IlluminOss Medical11.03.16
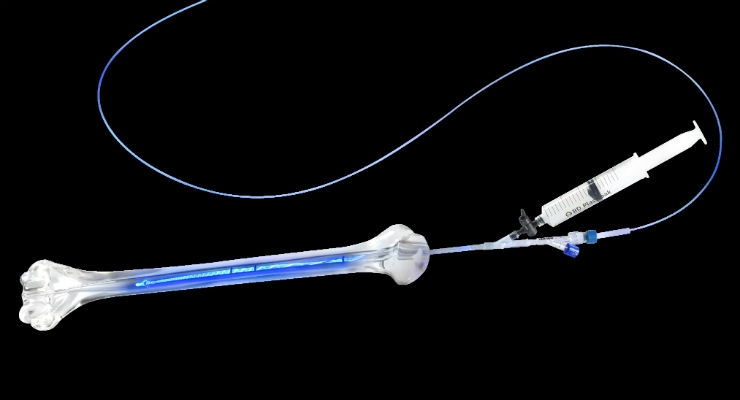
IlluminOss Medical, a privately-held, commercial stage medical device company focused on minimally invasive orthopedic fracture repair, has announced that Richard Terek, M.D., presented the first preliminary data from its U.S. pivotal clinical trial at the recent Musculoskeletal Tumor Society (MSTS) Meeting in Detroit. The IlluminOss System is the world’s only system of its kind that supports the treatment of fractures using patient-specific bone conforming intramedullary implants.
IlluminOss’ LightFix Trial is a multi-center study of the IlluminOss System for the treatment of impending and actual pathological fractures in the humerus from metastatic bone disease Preliminary results from the U.S. trial measured pain and functional improvement, as well as evaluated safety and performance.
“The investigators found that the technical nature and attributes of the product were viewed to be easier and faster to use, with no limitations to the use of adjunctive hardware,” said Dr. Richard Terek.
Dr. Terek, an orthopedic surgeon and musculoskeletal oncologist who specializes in reconstructive surgery and musculoskeletal oncology and serves as professor in the department of orthopedic surgery at The Warren Alpert Medical School of Brown University, used the IlluminOss System in the treatment of patients at Rhode Island Hospital and is the first to publicly present trial results.
“The preliminary results of the LightFix trial have been extremely positive in that the investigators found that the device was less invasive than many standard orthopedic implants. The early data suggests that the patients achieved a decrease in pain and an increase in return to function through the use of the IlluminOss implant,” said Dr. Terek. “We are encouraged with the results of the IlluminOss System and look forward to its availability in the USA. It will offer the U.S. market a new and much-needed alternative for the treatment of patients with metastatic bone disease and other complex fractures.”
The IlluminOss System was developed with the potential to provide better patient experiences and outcomes than currently used nails and plates. IlluminOss has been used since 2010 in the treatment of patients in international markets, where it has been observed to result in smaller incisions, shorter procedure times, and more rapid post-procedure patient mobility with reduced hospital stays and lower complication rates for patients.
“This first presentation of our clinical data is consistent with the positive results we have seen in Europe and is further substantiation of the merits of our platform’s capabilities and how they may benefit the orthopedic community,” said Manny Avila, CEO, IlluminOss. “We look forward to the presentation of the completed LightFix trial results and the opportunity to provide the product to a much broader patient base.”
IlluminOss’ LightFix Trial is a multi-center study of the IlluminOss System for the treatment of impending and actual pathological fractures in the humerus from metastatic bone disease Preliminary results from the U.S. trial measured pain and functional improvement, as well as evaluated safety and performance.
“The investigators found that the technical nature and attributes of the product were viewed to be easier and faster to use, with no limitations to the use of adjunctive hardware,” said Dr. Richard Terek.
Dr. Terek, an orthopedic surgeon and musculoskeletal oncologist who specializes in reconstructive surgery and musculoskeletal oncology and serves as professor in the department of orthopedic surgery at The Warren Alpert Medical School of Brown University, used the IlluminOss System in the treatment of patients at Rhode Island Hospital and is the first to publicly present trial results.
“The preliminary results of the LightFix trial have been extremely positive in that the investigators found that the device was less invasive than many standard orthopedic implants. The early data suggests that the patients achieved a decrease in pain and an increase in return to function through the use of the IlluminOss implant,” said Dr. Terek. “We are encouraged with the results of the IlluminOss System and look forward to its availability in the USA. It will offer the U.S. market a new and much-needed alternative for the treatment of patients with metastatic bone disease and other complex fractures.”
The IlluminOss System was developed with the potential to provide better patient experiences and outcomes than currently used nails and plates. IlluminOss has been used since 2010 in the treatment of patients in international markets, where it has been observed to result in smaller incisions, shorter procedure times, and more rapid post-procedure patient mobility with reduced hospital stays and lower complication rates for patients.
“This first presentation of our clinical data is consistent with the positive results we have seen in Europe and is further substantiation of the merits of our platform’s capabilities and how they may benefit the orthopedic community,” said Manny Avila, CEO, IlluminOss. “We look forward to the presentation of the completed LightFix trial results and the opportunity to provide the product to a much broader patient base.”