Business Wire11.29.16
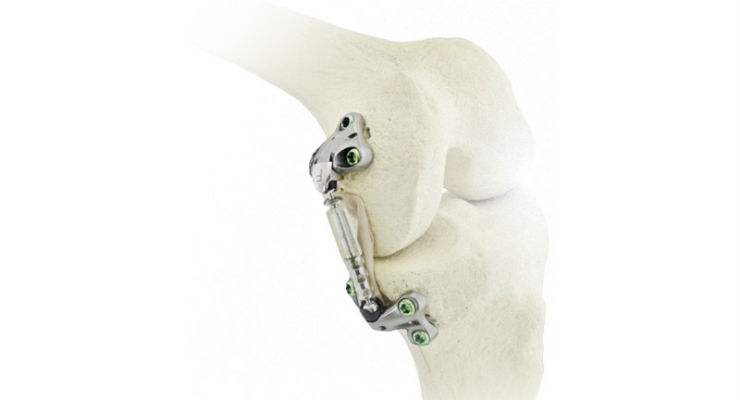
Moximed Inc. announced today that the first patient has been treated in an FDA-approved IDE study for its latest generation unicompartmental unloading implant, the Atlas System. The Atlas System builds on the company’s eight years of clinical success with joint unloading implants for medial knee osteoarthritis (OA).
“I’m pleased to treat the first patient in the Atlas IDE study. I see in my practice a tremendous number of younger, early OA patients who are seeking an alternative to arthroplasty that will allow them to maintain a highly active lifestyle,” noted Andreas Gomoll, M.D., Associate Professor of Orthopaedic Surgery at Harvard Medical School and Director of the Orthopaedic Program at the Brigham and Women’s Hospital Center for Regenerative Medicine. “Load distribution plays an important role in early osteoarthritis, and these patients could potentially benefit from a joint unloading procedure.”
The Principal US Investigator of the previous Moximed clinical study (KineSpring System), Jack Farr, M.D., Director of the OrthoIndy Cartilage Restoration Center of Indiana and the OrthoIndy Sports Medicine Fellowship Program remarked, "The Atlas System provides the same 30 lbs. of joint unloading with a significantly smaller implant and anatomically-guided surgical technique. Patients intuitively understand the concept of a shock absorber and are excited about a procedure that preserves their own anatomy without the bone cuts associated with joint replacement." He went on to say, “I am looking forward to enrolling my first patients in the Atlas Study." His site in Indianapolis, Ind. is now open and recruiting patients.
“Beginning a US study with the Atlas System is a noteworthy accomplishment for the company,” stated Moximed CEO Kevin Sidow. “Following on the maturing data from the pivotal study of our previous generation device, we are now excited to study the Atlas System for US patients in the clinical setting. We anticipate the Atlas IDE study will reproduce the positive outcomes and safety data presented from the first international studies of the device.”
“I’m pleased to treat the first patient in the Atlas IDE study. I see in my practice a tremendous number of younger, early OA patients who are seeking an alternative to arthroplasty that will allow them to maintain a highly active lifestyle,” noted Andreas Gomoll, M.D., Associate Professor of Orthopaedic Surgery at Harvard Medical School and Director of the Orthopaedic Program at the Brigham and Women’s Hospital Center for Regenerative Medicine. “Load distribution plays an important role in early osteoarthritis, and these patients could potentially benefit from a joint unloading procedure.”
The Principal US Investigator of the previous Moximed clinical study (KineSpring System), Jack Farr, M.D., Director of the OrthoIndy Cartilage Restoration Center of Indiana and the OrthoIndy Sports Medicine Fellowship Program remarked, "The Atlas System provides the same 30 lbs. of joint unloading with a significantly smaller implant and anatomically-guided surgical technique. Patients intuitively understand the concept of a shock absorber and are excited about a procedure that preserves their own anatomy without the bone cuts associated with joint replacement." He went on to say, “I am looking forward to enrolling my first patients in the Atlas Study." His site in Indianapolis, Ind. is now open and recruiting patients.
“Beginning a US study with the Atlas System is a noteworthy accomplishment for the company,” stated Moximed CEO Kevin Sidow. “Following on the maturing data from the pivotal study of our previous generation device, we are now excited to study the Atlas System for US patients in the clinical setting. We anticipate the Atlas IDE study will reproduce the positive outcomes and safety data presented from the first international studies of the device.”