Business Wire01.10.17
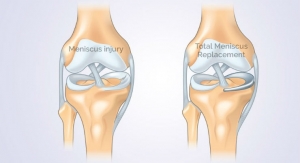
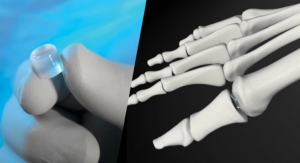
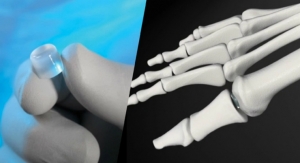
Cartiva Inc., a developer of products for the treatment of cartilage damage and osteoarthritis, announced today that it has secured $8.5 million in an oversubscribed Series E financing led by New Enterprise Associates (NEA) with participation by Windham Venture Partners and other returning investors. Proceeds from the financing will support the Company’s commercialization of its Cartiva Synthetic Cartilage Implant (SCI), the first Premarket Approval (PMA) approved alternative to fusion for arthritis of the big toe joint, and for general corporate purposes.
“With compelling Level I clinical data, Cartiva SCI is an exciting new product in the lower extremities market, the fastest growing segment of orthopedics. We are pleased to continue our partnership with Cartiva and to support their progress in making Cartiva SCI widely available as a preferred motion-preserving treatment option,” said Justin Klein, M.D., J.D., NEA partner and a member of Cartiva’s board of directors.
The Company also announced that it has expanded its existing debt facility with Silicon Valley Bank, providing for additional optional term loan debt and the addition of an accounts receivable revolving credit facility. Scott McCarty, Director at Silicon Valley Bank said, “We have had a long-term relationship with Cartiva and are pleased to offer them the financial services they need to support their rapidly expanding business.”
“With compelling Level I clinical data, Cartiva SCI is an exciting new product in the lower extremities market, the fastest growing segment of orthopedics. We are pleased to continue our partnership with Cartiva and to support their progress in making Cartiva SCI widely available as a preferred motion-preserving treatment option,” said Justin Klein, M.D., J.D., NEA partner and a member of Cartiva’s board of directors.
The Company also announced that it has expanded its existing debt facility with Silicon Valley Bank, providing for additional optional term loan debt and the addition of an accounts receivable revolving credit facility. Scott McCarty, Director at Silicon Valley Bank said, “We have had a long-term relationship with Cartiva and are pleased to offer them the financial services they need to support their rapidly expanding business.”