PR Newswire01.26.17
DePuy Synthes, part of the Johnson & Johnson Family of Companies, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the VIPER and EXPEDIUM Fenestrated Screw Systems. When used in conjunction with CONFIDENCE High Viscosity Spinal Cement, the screws are intended to restore the integrity of the spinal column in patients with advanced stage spinal tumors. The VIPER and EXPEDIUM Fenestrated Screw Systems may be used in open or percutaneous spinal fusion surgery.
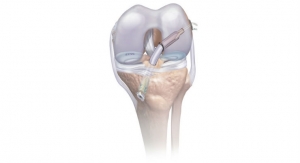
Metastatic spine disease accounts for 10 percent to 30 percent of new cancer diagnoses annually.1 Surgical fixation with pedicle screws may be used as palliative care to stabilize the spine, help reduce pain and help keep the patient mobile.1 The VIPER and EXPEDIUM Fenestrated Screws are designed with a hollow shaft, or cannulation. This design along with holes called fenestrations above the screw tip enable controlled delivery of CONFIDENCE High Viscosity Spinal Cement into the vertebra to provide immediate screw fixation.
"Metastatic disease in the spine can be severely painful and limiting for patients who are really trying to maintain quality of life, and there is a significant need for spinal implants that enhance stability in metastatic bone disease so that these patients can continue to function freely," said William C. Horton, M.D., vice president of research & development, DePuy Synthes Spine. "We designed these fenestrated screw systems to help address those needs, and to facilitate minimally invasive solutions for patients suffering from this disease."
The VIPER Fenestrated Screws are compatible with the VIPER and EXPEDIUM 5.5 Spine Systems and the EXPEDIUM Fenestrated Screws are compatible with the EXPEDIUM VERSE Spinal System. Both fenestrated screw systems are anticipated to be available in the United States in mid-2017.
References
1Dunning, E.C, et al. Complications in the management of metastatic spinal disease. World J Orthop. 2012 Aug 18; 3(8): 114–121.
Metastatic spine disease accounts for 10 percent to 30 percent of new cancer diagnoses annually.1 Surgical fixation with pedicle screws may be used as palliative care to stabilize the spine, help reduce pain and help keep the patient mobile.1 The VIPER and EXPEDIUM Fenestrated Screws are designed with a hollow shaft, or cannulation. This design along with holes called fenestrations above the screw tip enable controlled delivery of CONFIDENCE High Viscosity Spinal Cement into the vertebra to provide immediate screw fixation.
"Metastatic disease in the spine can be severely painful and limiting for patients who are really trying to maintain quality of life, and there is a significant need for spinal implants that enhance stability in metastatic bone disease so that these patients can continue to function freely," said William C. Horton, M.D., vice president of research & development, DePuy Synthes Spine. "We designed these fenestrated screw systems to help address those needs, and to facilitate minimally invasive solutions for patients suffering from this disease."
The VIPER Fenestrated Screws are compatible with the VIPER and EXPEDIUM 5.5 Spine Systems and the EXPEDIUM Fenestrated Screws are compatible with the EXPEDIUM VERSE Spinal System. Both fenestrated screw systems are anticipated to be available in the United States in mid-2017.
References
1Dunning, E.C, et al. Complications in the management of metastatic spinal disease. World J Orthop. 2012 Aug 18; 3(8): 114–121.