ChoiceSpine02.08.17
Spinal implant developer ChoiceSpine is launching HARRIER, an anterior lumbar interbody fusion system. The company received U.S. Food and Drug Administration 510(k) clearance for the HARRIER in November 2016.
The device is the first of four new products the company is launching in 2017.
“HARRIER fills a long-needed gap in our product portfolio,” said Anderson Collins, ChoiceSpine’s director of business development. “We have had strong growth over the past four years, which has been a function of two key drivers—bringing new products to market and working the right distributor partners. Adding HARRIER to our portfolio gives us another revenue source that our distributor and surgeon partners can utilize to improve patients’ lives.”
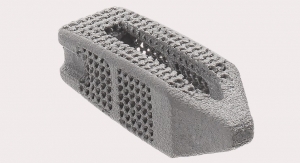
The ChoiceSpine HARRIER anterior lumbar interbody fusion system consists of PEEK (polyether ether ketone) implants meant to be used with supplemental fixation. The devices are offered in a variety of sizes to accommodate various anatomies a feature large bone graft windows to maximize bone in-growth. The surfaces of the interbody have machined edges meant to engage the vertebral endplates and prevent expulsion.
“Our product pipeline is full of products we plan to launch over the next two years. HARRIER and other new products will help sustain the growth we have experienced over the past few years," added Steve Ainsworth, ChoiceSpine’s vice president of R&D. "Our R&D team has worked diligently with our distributor and surgeon partners to roll out HARRIER and we are confident it will be received well in the market."
ChoiceSpine is based in Knoxville, Tenn. Founded in 2006, the company has more than 45 employees and is majority owned by Henson and Altshuler. Last week, the company announced it had acquired the spinal assets of Exactech, a developer and producer of bone and joint restoration products for extremities, hip, knee and spine.
The device is the first of four new products the company is launching in 2017.
“HARRIER fills a long-needed gap in our product portfolio,” said Anderson Collins, ChoiceSpine’s director of business development. “We have had strong growth over the past four years, which has been a function of two key drivers—bringing new products to market and working the right distributor partners. Adding HARRIER to our portfolio gives us another revenue source that our distributor and surgeon partners can utilize to improve patients’ lives.”
The ChoiceSpine HARRIER anterior lumbar interbody fusion system consists of PEEK (polyether ether ketone) implants meant to be used with supplemental fixation. The devices are offered in a variety of sizes to accommodate various anatomies a feature large bone graft windows to maximize bone in-growth. The surfaces of the interbody have machined edges meant to engage the vertebral endplates and prevent expulsion.
“Our product pipeline is full of products we plan to launch over the next two years. HARRIER and other new products will help sustain the growth we have experienced over the past few years," added Steve Ainsworth, ChoiceSpine’s vice president of R&D. "Our R&D team has worked diligently with our distributor and surgeon partners to roll out HARRIER and we are confident it will be received well in the market."
ChoiceSpine is based in Knoxville, Tenn. Founded in 2006, the company has more than 45 employees and is majority owned by Henson and Altshuler. Last week, the company announced it had acquired the spinal assets of Exactech, a developer and producer of bone and joint restoration products for extremities, hip, knee and spine.