Business Wire02.22.17
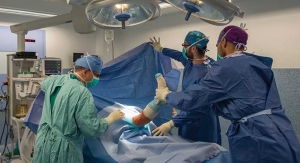
Wenzel Spine Inc., a medical technology company focused on providing minimally invasive solutions for the treatment of spinal disorders, today announced that it has completed the acquisition of the PrimaLOK SP Interspinous Fusion System and PrimaLOK FF Facet Fixation System from OsteoMed, LLC.
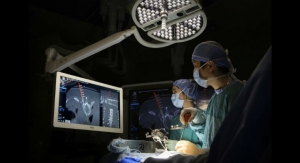
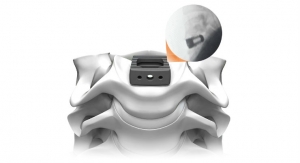
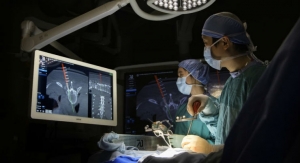
The PrimaLOK SP & FF platforms include a polyaxial interspinous process device and percutaneous facet screw system designed for MIS applications in treatment of lumbar spinal disorders. These patented technologies are designed to provide surgeons an expanded MIS solution when used in conjunction with the stand-alone, expandable, VariLift-LX interbody fusion device.
“This acquisition provides our distributors and surgeons a powerful combination of MIS solutions. When paired with our VariLift-LX stand-alone, expandable, interbody technology, surgeons now have the ability to provide supplemental fixation, when needed, without having to revert to pedicle screw constructs,” said Chad Neely, president and CEO of Wenzel Spine. “In addition, this expands our current product portfolio while strengthening our development pipeline and demonstrating our commitment to the development of MIS solutions to our distribution and surgeon partners.”
Dr. Charles Gordon, the inventor of the PrimaLOK SP System, and founder of the Texas Spine & Joint Hospital, commented, “I am pleased that Wenzel Spine has added the PrimaLOK Systems to their product portfolio. We are very happy to be partnered with a Spine focused company and believe Wenzel Spine is the ideal partner to bring the full potential of the PrimaLOK Systems to market.” Dr. Gordon further commented, “There are tremendous MIS opportunities in combining the advantages of the PrimaLOK and VariLift-LX technologies.”
Wenzel Spine plans to integrate the PrimaLOK platforms into the pipeline of MIS solutions currently being developed. The company has completed a limited US release of the PrimaLOK SP & FF Systems and expects to offer wide US release of the products in the second quarter of 2017.
The PrimaLOK SP & FF platforms include a polyaxial interspinous process device and percutaneous facet screw system designed for MIS applications in treatment of lumbar spinal disorders. These patented technologies are designed to provide surgeons an expanded MIS solution when used in conjunction with the stand-alone, expandable, VariLift-LX interbody fusion device.
“This acquisition provides our distributors and surgeons a powerful combination of MIS solutions. When paired with our VariLift-LX stand-alone, expandable, interbody technology, surgeons now have the ability to provide supplemental fixation, when needed, without having to revert to pedicle screw constructs,” said Chad Neely, president and CEO of Wenzel Spine. “In addition, this expands our current product portfolio while strengthening our development pipeline and demonstrating our commitment to the development of MIS solutions to our distribution and surgeon partners.”
Dr. Charles Gordon, the inventor of the PrimaLOK SP System, and founder of the Texas Spine & Joint Hospital, commented, “I am pleased that Wenzel Spine has added the PrimaLOK Systems to their product portfolio. We are very happy to be partnered with a Spine focused company and believe Wenzel Spine is the ideal partner to bring the full potential of the PrimaLOK Systems to market.” Dr. Gordon further commented, “There are tremendous MIS opportunities in combining the advantages of the PrimaLOK and VariLift-LX technologies.”
Wenzel Spine plans to integrate the PrimaLOK platforms into the pipeline of MIS solutions currently being developed. The company has completed a limited US release of the PrimaLOK SP & FF Systems and expects to offer wide US release of the products in the second quarter of 2017.