Business Wire03.08.17
Vertiflex Inc., a developer of advanced, minimally invasive interventions for spinal stenosis, today announced completion of a $40M financing round. New investors, Endeavour Vision and H.I.G. BioHealth Partners led the financing alongside existing investors, New Enterprise Associates; Thomas, McNerney & Partners; and Alta Partners. Proceeds from the financing will primarily be used to fund U.S. commercial expansion of the company’s Superion Indirect Decompression System, a minimally invasive spinal implant designed to treat moderate lumbar spinal stenosis, a painful and often debilitating condition that affects an estimated 500,000 new patients every year in the United States.
“Vertiflex has seen tremendous early success in the commercialization of the Superion System,” said Earl Fender, president and CEO of Vertiflex. “With favorable long-term clinical outcomes, a new Category I AMA CPT code and broad reimbursement in place, adoption of Superion has continued at a rapid and steady pace, driven by significant interest from both patients and treating physicians.”
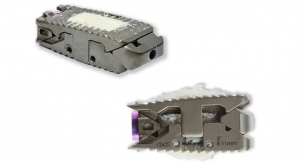
Once implanted, Superion is intended to reduce pressure on the affected nerves and allow patients to return to a more active lifestyle. Following completion of a successful 391 patient randomized controlled trial, the Superion System received Premarket Approval (PMA) from the U.S. Food and Drug Administration (FDA), and starting January 2017 the system is described by a new American Medical Association (AMA) Current Procedural Terminology (CPT) Category I code.
“I’m also excited to see continued momentum with our current investors who have steadfastly supported the company to date, and look forward to working closely with our new investors to realize the potential for the Superion System, as we further invest in the commercial expansion of an important treatment option for physicians and patients,” Fender added.
To accommodate its accelerating growth, Vertiflex recently relocated its corporate headquarters from San Clemente, Calif., to a larger facility in Carlsbad, Calif. The new location includes expanded distribution capability, and a state-of-the-art physician education center, as part of the company’s commitment to supporting physicians by providing didactic as well as hands-on cadaver training with Superion.
Nick Shamie, M.D., Chief of Orthopedic Spine Surgery and Professor of Orthopedic Surgery and Neurosurgery at UCLA School of Medicine, stated, “The Superion System offers patients a minimally invasive solution to treat leg pain associated with spinal stenosis. The implant, placed through a small tube the size of a dime, does not deconstruct any of the anatomical elements and provides immediate relief. I incorporated this treatment into my practice after seeing the clinical data from the FDA IDE clinical trial. The safety, efficacy, and five-year durability that the data presented made it a procedure I can stand behind.”
“Vertiflex has seen tremendous early success in the commercialization of the Superion System,” said Earl Fender, president and CEO of Vertiflex. “With favorable long-term clinical outcomes, a new Category I AMA CPT code and broad reimbursement in place, adoption of Superion has continued at a rapid and steady pace, driven by significant interest from both patients and treating physicians.”
Once implanted, Superion is intended to reduce pressure on the affected nerves and allow patients to return to a more active lifestyle. Following completion of a successful 391 patient randomized controlled trial, the Superion System received Premarket Approval (PMA) from the U.S. Food and Drug Administration (FDA), and starting January 2017 the system is described by a new American Medical Association (AMA) Current Procedural Terminology (CPT) Category I code.
“I’m also excited to see continued momentum with our current investors who have steadfastly supported the company to date, and look forward to working closely with our new investors to realize the potential for the Superion System, as we further invest in the commercial expansion of an important treatment option for physicians and patients,” Fender added.
To accommodate its accelerating growth, Vertiflex recently relocated its corporate headquarters from San Clemente, Calif., to a larger facility in Carlsbad, Calif. The new location includes expanded distribution capability, and a state-of-the-art physician education center, as part of the company’s commitment to supporting physicians by providing didactic as well as hands-on cadaver training with Superion.
Nick Shamie, M.D., Chief of Orthopedic Spine Surgery and Professor of Orthopedic Surgery and Neurosurgery at UCLA School of Medicine, stated, “The Superion System offers patients a minimally invasive solution to treat leg pain associated with spinal stenosis. The implant, placed through a small tube the size of a dime, does not deconstruct any of the anatomical elements and provides immediate relief. I incorporated this treatment into my practice after seeing the clinical data from the FDA IDE clinical trial. The safety, efficacy, and five-year durability that the data presented made it a procedure I can stand behind.”