Bioventus03.17.17
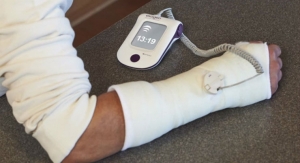
Bioventus, a global provider of orthobiologics, is commissioning a series of real-world evidence, direct-to-patient studies to further validate the ability of its EXOGEN Ultrasound Bone Healing System to mitigate the risk of a fracture progressing to nonunion in the presence of known risk factors. Approved by PMA by the U.S. Food and Drug Administration in 1994, EXOGEN has provided treatment to more than 1 million patients worldwide for more than 20 years and has a long clinical history. The product uses safe, effective low-intensity pulsed ultrasound (LIPUS) to help stimulate the body’s natural healing process.1
Recent publications analyzing data from a large registry of almost 8,000 fractures treated with EXOGEN suggest that the device supports healing fractures in patients despite the presence of associated comorbidities or medication use.2,3,4 This new clinical research, known as the Bioventus Observational Non-interventional EXOGEN Studies (BONES), will build on this evidence and supplement the product’s broad body of clinical knowledge in a prospective population-based innovative clinical development program.
“BONES represents a significant investment in developing epidemiologically grounded rigorous clinical evidence to support use of EXOGEN in fractures at risk, and to underscore the product’s clinical utility in mitigating the risk of nonunions, a highly debilitating and costly condition,” said Alessandra Pavesio, senior vice president and chief science officer for Bioventus. “It will build upon knowledge gained from extensive research conducted by Bioventus and recently published in JAMA Surgery, in over 700,000 fracture patients that has identified 40-plus factors which place a patient at an increased risk of progression to a nonunion.”5
Pavesio told Orthopedic Design & Technology that the study will focus on three bones in the upper and lower extremities: the scaphoid, metatarsal, and tibia. The three were chosen for their different shapes and potential healing complications. Study subjects will be musculoskeletally mature adults between 18 and 65 years old.
"EXOGEN is a very important product for us," Pavesio said. "We want to develop data that proves EXOGEN mitigates the risk of nonunion fractures and also shows the economic benefits of the product. We're very excited about the study."
Bioventus is an orthobiologics company that develops clinically proven, cost-effective products that help people heal quickly and safely. Durham, N.C.-based Bioventus has two product portfolios for orthobiologics, Bioventus Active Healing Therapies and Bioventus Surgical that make it a global leader in active orthopaedic healing. Its EXOGEN Ultrasound Bone Healing System is the top-prescribed bone healing system in the United States and is the only FDA-approved bone healing device that uses safe, effective ultrasound to stimulate the body’s natural healing process.
Bioventus, the Bioventus logo, and EXOGEN are registered trademarks of Bioventus LLC.
References:
1. Azuma Y, Ito M, Harada Y, Takagi H, Ohta T, Jingushi S. Low-intensity pulsed ultrasound accelerates rat femoral fracture healing by acting on the various cellular reactions in the fracture callus.J Bone Miner Res. 2001; 16(4):671-680.
2. Nolte P, Anderson R, Strauss E, Hu L, J Jones, RG Steen. 2016. Heal rate of metatarsal fractures: A propensity-matching study of low-intensity pulsed ultrasound (LIPUS) vs. surgical and other treatments. Injury. 2017 47(11):2584-2590.
3. Zura R, G Della Rocca, S Mehta, A Harrison, C Brodie, J Jones, RG Steen. 2015. Treatment of chronic (> 1 year) fracture nonunion: Heal rate in a cohort of 767 patients treated with low-intensity pulsed ultrasound (LIPUS). Injury 46:2036-2041.
4. Zura R, S Mehta, G Della Rocca, J Jones, RG Steen. 2015. A cohort study of 4,190 patients treated with low-intensity pulsed ultrasound (LIPUS): Findings in the elderly versus all patients. BMC Musculoskel. Dis. 16:45.
5. Zura R, Xiong Z, Einhorn T, Watson JT, Ostrum RF, Prayson MJ, Della Rocca GJ, Mehta S, McKinley T, Wang Z, Steen RG. Epidemiology of Fracture Nonunion in 18 Human Bones, JAMA Surger 2016: e162775. doi:10.1001/jamasurg.2016.2775.
Recent publications analyzing data from a large registry of almost 8,000 fractures treated with EXOGEN suggest that the device supports healing fractures in patients despite the presence of associated comorbidities or medication use.2,3,4 This new clinical research, known as the Bioventus Observational Non-interventional EXOGEN Studies (BONES), will build on this evidence and supplement the product’s broad body of clinical knowledge in a prospective population-based innovative clinical development program.
“BONES represents a significant investment in developing epidemiologically grounded rigorous clinical evidence to support use of EXOGEN in fractures at risk, and to underscore the product’s clinical utility in mitigating the risk of nonunions, a highly debilitating and costly condition,” said Alessandra Pavesio, senior vice president and chief science officer for Bioventus. “It will build upon knowledge gained from extensive research conducted by Bioventus and recently published in JAMA Surgery, in over 700,000 fracture patients that has identified 40-plus factors which place a patient at an increased risk of progression to a nonunion.”5
Pavesio told Orthopedic Design & Technology that the study will focus on three bones in the upper and lower extremities: the scaphoid, metatarsal, and tibia. The three were chosen for their different shapes and potential healing complications. Study subjects will be musculoskeletally mature adults between 18 and 65 years old.
"EXOGEN is a very important product for us," Pavesio said. "We want to develop data that proves EXOGEN mitigates the risk of nonunion fractures and also shows the economic benefits of the product. We're very excited about the study."
Bioventus is an orthobiologics company that develops clinically proven, cost-effective products that help people heal quickly and safely. Durham, N.C.-based Bioventus has two product portfolios for orthobiologics, Bioventus Active Healing Therapies and Bioventus Surgical that make it a global leader in active orthopaedic healing. Its EXOGEN Ultrasound Bone Healing System is the top-prescribed bone healing system in the United States and is the only FDA-approved bone healing device that uses safe, effective ultrasound to stimulate the body’s natural healing process.
Bioventus, the Bioventus logo, and EXOGEN are registered trademarks of Bioventus LLC.
References:
1. Azuma Y, Ito M, Harada Y, Takagi H, Ohta T, Jingushi S. Low-intensity pulsed ultrasound accelerates rat femoral fracture healing by acting on the various cellular reactions in the fracture callus.J Bone Miner Res. 2001; 16(4):671-680.
2. Nolte P, Anderson R, Strauss E, Hu L, J Jones, RG Steen. 2016. Heal rate of metatarsal fractures: A propensity-matching study of low-intensity pulsed ultrasound (LIPUS) vs. surgical and other treatments. Injury. 2017 47(11):2584-2590.
3. Zura R, G Della Rocca, S Mehta, A Harrison, C Brodie, J Jones, RG Steen. 2015. Treatment of chronic (> 1 year) fracture nonunion: Heal rate in a cohort of 767 patients treated with low-intensity pulsed ultrasound (LIPUS). Injury 46:2036-2041.
4. Zura R, S Mehta, G Della Rocca, J Jones, RG Steen. 2015. A cohort study of 4,190 patients treated with low-intensity pulsed ultrasound (LIPUS): Findings in the elderly versus all patients. BMC Musculoskel. Dis. 16:45.
5. Zura R, Xiong Z, Einhorn T, Watson JT, Ostrum RF, Prayson MJ, Della Rocca GJ, Mehta S, McKinley T, Wang Z, Steen RG. Epidemiology of Fracture Nonunion in 18 Human Bones, JAMA Surger 2016: e162775. doi:10.1001/jamasurg.2016.2775.