PR Newswire04.12.17
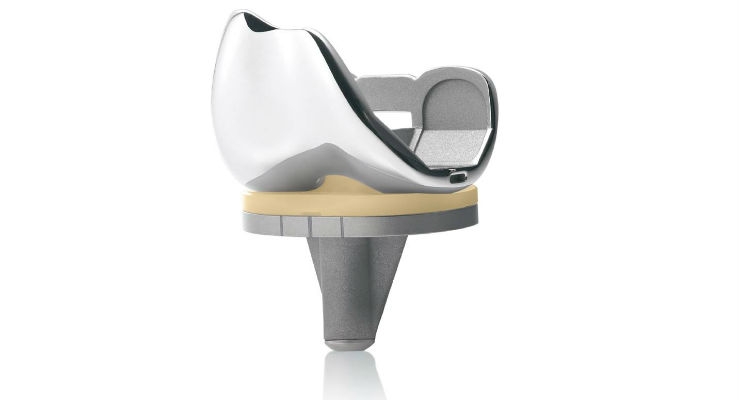
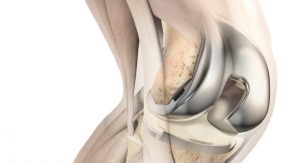
United Orthopedic Corporation (UOC), an international designer, manufacturer, and distributor of innovative orthopedic implants and instruments, has announced that the U.S. Food and Drug Administration (FDA) cleared its E-XPE polyethylene knee insert. The knee insert is designed to provide patients with knee replacements with reduced risk of oxidation.1
"Clearance of our E-XPE polyethylene knee insert represents an important milestone in our knee portfolio," said Calvin Lin, president of United Orthopedic Corporation USA. "Oxidation continues to be a concern for surgeons. The E-XPE polyethylene is resistant to oxidation and performs extremely well with respect to abrasive wear resistance."
The E-XPE is the new generation of highly cross-linked polyethylene blended with 0.1 percent (w/w) vitamin E to enhance wear resistance without compromising oxidative stability and mechanical properties.
UOC's vertically integrated manufacturing process is unique, enabling the company to control the production cycle of their implants, from initial design to distribution in each of its manufacturing facilities. By doing so, UOC is capable of ensuring a stable, quality product supply and a remarkable level of customization to meet the needs of patients.
More than 600,000 knee replacements are performed each year in the United States.2
References
1 Bellare, A. et al. Journal of Applied Polymer Science (2016). Oxidation Resistance and Abrasive Wear Resistance of Vitamin E Stabilized Radiation Crosslinked Ultra-High Molecular Weight Polyethylene, DOI:10.1002/APP.44125.
2 American Academy of Orthopedic Surgeons website. Beyond Surgery Day: The Full Impact of Knee Replacement. Accessed April 10, 2017 from: http://www.anationinmotion.org/value/knee.
"Clearance of our E-XPE polyethylene knee insert represents an important milestone in our knee portfolio," said Calvin Lin, president of United Orthopedic Corporation USA. "Oxidation continues to be a concern for surgeons. The E-XPE polyethylene is resistant to oxidation and performs extremely well with respect to abrasive wear resistance."
The E-XPE is the new generation of highly cross-linked polyethylene blended with 0.1 percent (w/w) vitamin E to enhance wear resistance without compromising oxidative stability and mechanical properties.
UOC's vertically integrated manufacturing process is unique, enabling the company to control the production cycle of their implants, from initial design to distribution in each of its manufacturing facilities. By doing so, UOC is capable of ensuring a stable, quality product supply and a remarkable level of customization to meet the needs of patients.
More than 600,000 knee replacements are performed each year in the United States.2
References
1 Bellare, A. et al. Journal of Applied Polymer Science (2016). Oxidation Resistance and Abrasive Wear Resistance of Vitamin E Stabilized Radiation Crosslinked Ultra-High Molecular Weight Polyethylene, DOI:10.1002/APP.44125.
2 American Academy of Orthopedic Surgeons website. Beyond Surgery Day: The Full Impact of Knee Replacement. Accessed April 10, 2017 from: http://www.anationinmotion.org/value/knee.