PR Newswire04.17.17
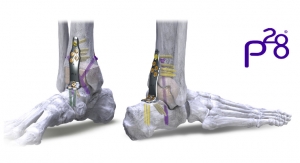
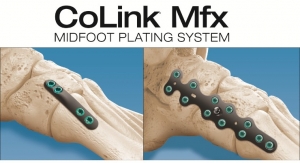
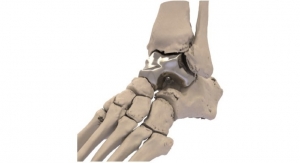
Additive Orthopaedics, LLC., an early stage orthopedic device company, announced that is has received FDA 510(k) clearance for its 3D printed Locking Lattice Plating System, to address stabilization and fusion of fractures, osteotomies, and arthrodesis of small bones.
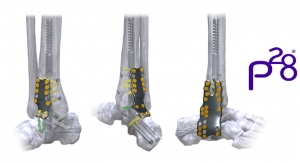
According to Greg Kowalczyk, president of Additive Orthopaedics, "We are excited to be one of the first companies to leverage the geometric flexibility, clinical advantages and manufacturing cost benefits of additive manufacturing in the orthopedic plating market. These plates can be implanted either alone with locking or non-locking screws, or in conjunction with our 3D printed bone segments through the use of a connection screw. This allows the surgeon to mix and match any wedge and plate combination for various deformities, complex revisions, or other limb salvage procedures."
This is the company's third 510(k) clearance leveraging additive manufacturing and fifth product line, which includes their 3D printed core products, biologics, and customs for limb salvage and complex revision cases. Surgeons have implanted over 300 of their individual devices since the first full commercial product launch at the end of 2016. The Company has recently closed a Series A Round of $1M.
According to Greg Kowalczyk, president of Additive Orthopaedics, "We are excited to be one of the first companies to leverage the geometric flexibility, clinical advantages and manufacturing cost benefits of additive manufacturing in the orthopedic plating market. These plates can be implanted either alone with locking or non-locking screws, or in conjunction with our 3D printed bone segments through the use of a connection screw. This allows the surgeon to mix and match any wedge and plate combination for various deformities, complex revisions, or other limb salvage procedures."
This is the company's third 510(k) clearance leveraging additive manufacturing and fifth product line, which includes their 3D printed core products, biologics, and customs for limb salvage and complex revision cases. Surgeons have implanted over 300 of their individual devices since the first full commercial product launch at the end of 2016. The Company has recently closed a Series A Round of $1M.