Business Wire05.10.17
Life Spine, a medical device company that designs, develops, manufactures, and markets products for the surgical treatment of spinal disorders, is pleased to announce the FDA 510(k) clearance of the CRANIAL FUSION System. This clearance expands the indications for utilizing SOLSTICE Polyaxial Screws into the cervical spine.
“With the clearance of the Cranial Fusion System, this is an exciting opportunity for use of our Solstice system screws for posterior cervical fusions,” said Mariusz Knap, vice president of marketing for Life Spine. “We continue to focus on advancements that strive to improve surgical efficiencies, ease of use and reliability of posterior occipito-cervico-thoracic fixation of the spine, thus providing the highest value of care to our customers and patients.”
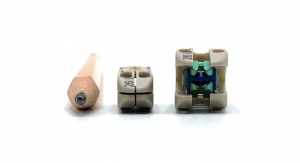
The CRANIAL FUSION System is a multiple component system comprised of titanium alloy, with a variety of occipital plates, occipital bone screws, polyaxial screws, hooks, connectors, rods, and locking caps.
SOLSTICE polyaxial screws used with the CRANIAL FUSION System come in 3.5, 4.0 and 4.5mm diameters. The conical polyaxial head angulation facilitates easy rod placement with minimal contouring, and the “friction head” feature maintains screw head position within the surgical wound.
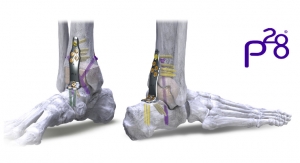
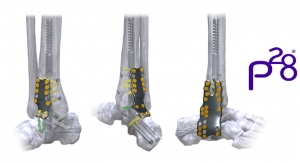
In order to achieve additional levels of fixation, The CRANIAL FUSION System may be connected to the NAUTILUS Thoracolumbar Pedicle Screw System using the 3.5/5.5mm titanium parallel connectors. The hooks and rods are also intended to provide stabilization to promote fusion following reduction of fracture/dislocation or trauma in the cervical/upper thoracic (C1-T3) spine.
“With the clearance of the Cranial Fusion System, this is an exciting opportunity for use of our Solstice system screws for posterior cervical fusions,” said Mariusz Knap, vice president of marketing for Life Spine. “We continue to focus on advancements that strive to improve surgical efficiencies, ease of use and reliability of posterior occipito-cervico-thoracic fixation of the spine, thus providing the highest value of care to our customers and patients.”
The CRANIAL FUSION System is a multiple component system comprised of titanium alloy, with a variety of occipital plates, occipital bone screws, polyaxial screws, hooks, connectors, rods, and locking caps.
SOLSTICE polyaxial screws used with the CRANIAL FUSION System come in 3.5, 4.0 and 4.5mm diameters. The conical polyaxial head angulation facilitates easy rod placement with minimal contouring, and the “friction head” feature maintains screw head position within the surgical wound.
In order to achieve additional levels of fixation, The CRANIAL FUSION System may be connected to the NAUTILUS Thoracolumbar Pedicle Screw System using the 3.5/5.5mm titanium parallel connectors. The hooks and rods are also intended to provide stabilization to promote fusion following reduction of fracture/dislocation or trauma in the cervical/upper thoracic (C1-T3) spine.