Business Wire05.19.17
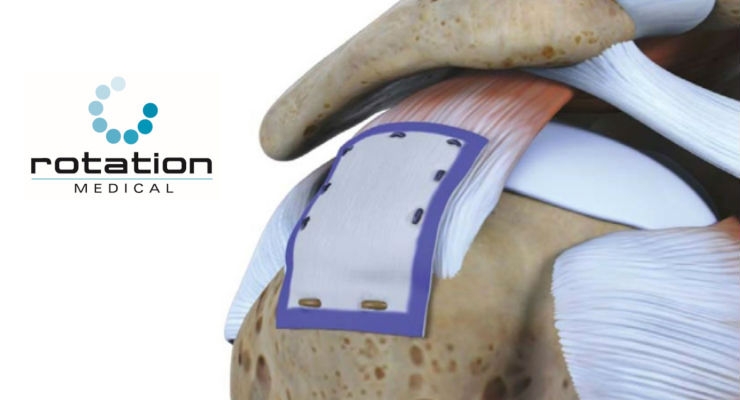
Rotation Medical Inc., a medical device company focused on developing new technologies to treat rotator cuff disease, announced initial results for the REBUILD registry of the company’s Bioinductive Implant at the Arthroscopy Association of North America (AANA) Annual Meeting. The REBUILD registry adds to the growing body of literature supporting the use of the Rotation Medical Bioinductive Implant as a novel treatment for rotator cuff injury.
REBUILD (Rotation MEdical BioindUctive ImpLant Database) is a prospective, non-randomized, multicenter registry designed to collect patient reported outcomes, including shoulder function, pain and quality of life after receiving the Bioinductive Implant. Interim results of the first 200 patients showed significantly less post-operative pain and use of narcotics, less sling time, faster return to function and better overall shoulder rating. The study will follow up to 300 patients across 20 study centers.
“Patients often opt out or delay surgery in the early stages of rotator cuff disease due to long and painful rehabilitation,” said Dr. Louis McIntyre, study investigator and orthopedic surgeon at Northwell Health Physician Partners Orthopaedic Institute at Sleepy Hollow, N.Y. “Initial results of the REBUILD registry demonstrate statistically significant improvements in several outcomes measures, including pain, function, and overall shoulder rating.”
Data in the REBUILD registry are being compared to data in a national orthopedic database using standard rotator cuff treatment. Patients in the REBUILD registry report feeling better six months after receiving the Bioinductive Implant than those who received standard treatment did at two years.
“Payors and providers are increasingly looking for data that demonstrate the effectiveness of medical devices on key health economic drivers,” said Martha Shadan, president and CEO of Rotation Medical. “With the results of the REBUILD registry, we believe our Bioinductive Implant is well-positioned to transform the treatment of rotator cuff disease, enabling patients to get back to work and other activities more quickly with less pain and use of narcotics than traditional rotator cuff repair.”
REBUILD (Rotation MEdical BioindUctive ImpLant Database) is a prospective, non-randomized, multicenter registry designed to collect patient reported outcomes, including shoulder function, pain and quality of life after receiving the Bioinductive Implant. Interim results of the first 200 patients showed significantly less post-operative pain and use of narcotics, less sling time, faster return to function and better overall shoulder rating. The study will follow up to 300 patients across 20 study centers.
“Patients often opt out or delay surgery in the early stages of rotator cuff disease due to long and painful rehabilitation,” said Dr. Louis McIntyre, study investigator and orthopedic surgeon at Northwell Health Physician Partners Orthopaedic Institute at Sleepy Hollow, N.Y. “Initial results of the REBUILD registry demonstrate statistically significant improvements in several outcomes measures, including pain, function, and overall shoulder rating.”
Data in the REBUILD registry are being compared to data in a national orthopedic database using standard rotator cuff treatment. Patients in the REBUILD registry report feeling better six months after receiving the Bioinductive Implant than those who received standard treatment did at two years.
“Payors and providers are increasingly looking for data that demonstrate the effectiveness of medical devices on key health economic drivers,” said Martha Shadan, president and CEO of Rotation Medical. “With the results of the REBUILD registry, we believe our Bioinductive Implant is well-positioned to transform the treatment of rotator cuff disease, enabling patients to get back to work and other activities more quickly with less pain and use of narcotics than traditional rotator cuff repair.”