EIN Presswire01.12.18
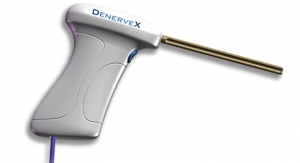
Bodycad has received a Health Canada medical device license and clearance to sell the Bodycad OnCall personalized cutting guides. Bodycad is the first Canadian manufacturer to receive a license from Health Canada for a personalized orthopedic bone resection device.
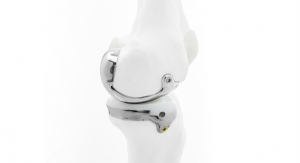
Bodycad’s OnCall Cutting Guide System is designed to optimize personalized restoration of difficult bone resection procedures such as deformity correction and oncology tumor resection. The system is based on proprietary 3D rendering of medical images of the patient’s anatomy. The device is delivered as a “procedure in box” that completely revolutionizes the way orthopedic applications are delivered and used in the operating room environment.
“The personalized bone resection is created only after proper acquisition of data from the patient on an individualized level,” said Etienne Belize, M.D., orthopedic surgeon and assistant professor at Laval University. “The benefit of a personalized device is the possibility of a better fit to the individual, less trauma to the soft tissue, and potentially a faster recovery overall.”
Bodycad uses proprietary imaging algorithms to rapidly produce a precise 3D image of the patient’s anatomy. Its suite of Personalized Restoration Software enables a seamless integration of the image to personalized solution called the PREP (personalized restoration evaluation process). The efficient and rapid process is designed to increase patient satisfaction while improving economic quality metrics.
“Our proprietary software is based on 20 years of research in anthropometric data and has been specifically developed and optimized for personalization of orthopedic surgical instruments, implants, and procedures.,” said Jean Robichaud, founder and CEO of Bodycad. “I am delighted to have Health Canada licensure and clearance to bring this important technological advancement to market. Our goal is to transform the way surgeons, patients and insurers think about the potential of mass customization to optimize patient care and bring true personalization to the orthopedic market place.”
Bodycad is a Quebec City-based developer and manufacturer of personalized orthopedic products. Its personalized restorations offer patients a high level of conformity to their unique anatomy, with the potential for greater comfort, fit and durability that make the pursuit of orthopedic perfection possible.
Bodycad’s OnCall Cutting Guide System is designed to optimize personalized restoration of difficult bone resection procedures such as deformity correction and oncology tumor resection. The system is based on proprietary 3D rendering of medical images of the patient’s anatomy. The device is delivered as a “procedure in box” that completely revolutionizes the way orthopedic applications are delivered and used in the operating room environment.
“The personalized bone resection is created only after proper acquisition of data from the patient on an individualized level,” said Etienne Belize, M.D., orthopedic surgeon and assistant professor at Laval University. “The benefit of a personalized device is the possibility of a better fit to the individual, less trauma to the soft tissue, and potentially a faster recovery overall.”
Bodycad uses proprietary imaging algorithms to rapidly produce a precise 3D image of the patient’s anatomy. Its suite of Personalized Restoration Software enables a seamless integration of the image to personalized solution called the PREP (personalized restoration evaluation process). The efficient and rapid process is designed to increase patient satisfaction while improving economic quality metrics.
“Our proprietary software is based on 20 years of research in anthropometric data and has been specifically developed and optimized for personalization of orthopedic surgical instruments, implants, and procedures.,” said Jean Robichaud, founder and CEO of Bodycad. “I am delighted to have Health Canada licensure and clearance to bring this important technological advancement to market. Our goal is to transform the way surgeons, patients and insurers think about the potential of mass customization to optimize patient care and bring true personalization to the orthopedic market place.”
Bodycad is a Quebec City-based developer and manufacturer of personalized orthopedic products. Its personalized restorations offer patients a high level of conformity to their unique anatomy, with the potential for greater comfort, fit and durability that make the pursuit of orthopedic perfection possible.