Implantable PEEK Polymers: A Decade of Progress in Spine
Device manufacturers continue to expand their PEEK product offerings.
Steven M. Kurtz, Drexel University
Within the past decade, implantable polyetheretherketone (PEEK) polymers have matured into an established and widely accepted family of biomaterials for spinal devices. In addition to
PEEK-based dynamic stabilization rods. Photo courtesy of Invibio Biomaterial Solutions. |
biocompatibility, biostability and compatibility with diagnostic imaging, these advanced thermoplastic polymers provide a range of mechanical properties that are well suited to the demanding environment of spinal implants. A consistent supply of PEEK—manufactured in accordance with stringent quality standards and backed by full raw material traceability—key to its wide use.
Implantable PEEK polymers are available today in an array of formulations, ranging from unfilled grades with varying molecular weight, to image-contrast and carbon fiber-reinforced grades. The first implantable unfilled PEEK polymer—PEEK-OPTIMA—was pioneered in 1999 by United Kingdom-based Invibio Biomaterial Solutions. Introduced by Invibio in 2007 to provide controlled visibility through X-ray, CT and MRI technologies, image-contrast grades offer tailored opacity that allows for easier post-operative device placement verification by surgeons and clear assessment of the healing site. Also launched by Invibio in 2007, carbon fiber-reinforced (CFR) grades provide significantly increased strength and stiffness as well as a modulus similar to that of cortical bone. CFR-PEEK grades recently have been evaluated as a bearing material in cervical disc arthroplasty.
In many respects, the growth in acceptance of PEEK biomaterials is mirrored by the expansion of the field of instrumented spine surgery.
In the United States, instrumented spinal fusion is the most common surgical procedure for chronic neck and back pain for patients who do not respond to conservative treatment.
In 1997, an estimated 215,000 Americans underwent spine fusion procedures for intractable pain, and by 2007 the annual number of fusions in the United States increased to 402,000.1 PEEK polymer-based devices are widely used for these procedures, and further adoption of PEEK biomaterials is forecasted in the future.
Historically, spinal fusion instrumentation was fabricated from metallic biomaterials, including stainless steel and titanium alloy, because of their strength and fatigue resistance.
However, one key drawback of these metallic implants is incompatibility with diagnostic imaging, including MRI and CT scans, which are crucial for visualizing changes to the spinal cord and vital soft tissue structures of the spine. Laboratory studies during the 1990s confirmed that PEEK implants had the requisite combination of strength, wear, creep and fatigue resistance to replace metallic biomaterials for spine implants.
PEEK for Spinal Fusion
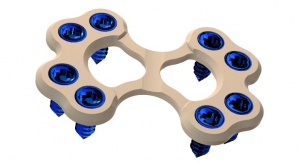
The first spinal instrumentation to replace metal with PEEK was an intervertebral fusion cage in 1999, made with PEEK-OPTIMA polymer from Invibio Biomaterial Solutions. The use of PEEK has since expanded as an alternative to metal implants not only with intervertebral cages, but also now with posterior and anterior instrumentation. Today, just a decade after its introduction, PEEK-based vertebral body replacements and interbody fusion devices are the standard of care for lumbar fusion.
Use of PEEK in cervical fusion is growing rapidly as well. Increased utilization of PEEK in cervical devices is supported by published literature reporting positive outcomes of PEEK-based devices for cervical fusion, including a clinical advantage over autograft alone or with plate and screw stabilization instrumentation across numerous different endpoints:2
• Improved spinal alignment and geometry 4,6, 7, 9,12
• Reduced hospital stays and decreased blood loss 4,7,8,10-12
• Decreased complication rates 4-8,10,11,12
• Good/excellent functional outcomesand improved patient satisfaction 3,5,10,11
• Excellent fusion rates 3,5,8-10
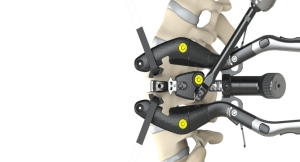
In the case of fusion systems employing rods, PEEK is intended to distribute stress more evenly to the anterior column compared with existing traditional, rigid metallic constructs consisting of plates, rods and screws. This transfer of stress may enable greater bone/end plate contact to encourage bone growth for fusion.
Minimally Invasive Fusion Surgery
Research on minimally invasive techniques, and the implants associated with these new procedures, is atthe forefront of current advances in spine surgery. PEEK increasingly is employed in minimally
17
invasive spine implants for fusion in an effort to reduce tissue dissection and nerve root retraction while preserving facet joints. The design and implantation techniqueof the Interfuse Interbody System from Vertebral Technologies Inc. in Minnetonka, Minn., is one example of a PEEK device that provides a large anterior lumbar interbody fusion-sized cage footprint via a less invasive approach.
Motion Preservation and Dynamic Stabilization
Currently, motion preservation and dynamic stabilization also are rapidly developing treatments in spinal surgery. Devices for these treatments include a variety of new implant technologies developed to preserve, limit or enhance motion of the spine. The application field has grown from its beginnings in interbody fusion to uses in posterior dynamic stabilization systems, nucleus replacement devices, cervical discs and interspinous devices. It is important to emphasize, however, that motion preservation and dynamic stabilization surgery, including those employing PEEK devices, are recently developed procedures with only a few years of clinical history in the United States.
Pedicle-Based Rod Systems for Non-fusion
PEEK has been specified in pedicle screw based rod systems. In some cases, PEEK rods are intended as an adjunct to fusion. Recently, there also has been increased interest in PEEK rods as dynamic stabilization devices, in which motion of the spine is stabilized without fusion. PEEK-based rods have been shown to exhibit sufficient fatigue resistance for both fusion and non-fusion stabilization procedures.
Interspinous Spacers
A natural progression from spinal fusion was the use of PEEK materials in interspinous spacers. The first use of PEEK was detailed in the Wallis system, which is intended to relieve or prevent low back pain that accompanies intervertebral segment instability by increasing stiffness of the mobile segment, and unloading the disc and facet joints.
The use of PEEK provides a lower modulus material and, in some designs, can result in a spacer 30 times more elastic than the first generation titanium devices reducing the risk of spinous process stress fractures.13
PEEK has been adopted in a number of new interspinous devices such as the X-Stop (Kyphon [now part of Medtronic Spine], Sunnyvale, Calif.), BacJac (Pioneer Surgical Technology; Marquette, Mich.), and Flexus (Globus Medical; Audubon, Pa.). These devices may offer benefits in terms of minimally invasive approaches without compromising on the large contact area necessary to ensure a minimal risk of subsidence.
Disc Arthroplasty
The consideration of PEEK as a material for use in spinal arthroplasty devices has been more recent. Traditionally, the spinal industry has looked to bearing materials used in other orthopedic sectors such
Courtesy of Invibio Biomaterial Solutions |
as ultra high molecular weight polyethylene (UHMWPE)/cobalt chromium molybdenum (CoCrMo) or metal on metal to provide low wear in spinal artificial discs. Wear studies have shown, however, that under certain spinal load and motions, all-polymer PEEK bearings provide a low-wear couple and that the material combination itself is relatively insensitive to gamma sterilization or the direction of motion.14
This originally was incorporated into the NUBAC nucleus replacement device and more recently into Pioneer Surgical Technology’s NuNec cervical artificial disc, a ball-in-socket design composed of PEEK–on–PEEK that uses a unique CAM interference screw locking mechanism for endplate fixation. Wear performance testing of NuNec compares favorably with many metal-on-metal or metal-on poly cervical discs.15 A recent paper, also investigating the wear performance of self-mating polyketone wear couples in ball-in-socket cervical disc devices, has demonstrated that CFR-PEEK materials offer a significant reduction in wear compared with UHMWPE/CoCrMo devices with a reported wear rate of 0.02±0.02 milligrams/million cycles compared with 1.0±0.1 milligrams/million cycles.
The clinical experience with PEEK disc replacements remains in its early stages.16 Although encouraging wear properties have been reported for all-polymer PEEK bearings in pre-clinical testing, longer term clinical results for this wear couple are eagerly anticipated to assess the viability of this wear couple in long-term bearing applications.
Future Directions for PEEK Advancements in Spine
PEEK biomaterials have more than a decade of successful clinical history in spinal fusion applications. PEEK polymers continue to influence the direction of spinal device design. A number of clinical trials and investigational device exemption studies are under way to evaluate PEEK in motion-preserving applications, such as in semi-rigid rod systems as well as in total-disc arthroplasty.
These studies will help define the future of motion preservation as a valued alternative to fusion.
With or without motion preservation, the need for advancement in biomaterials is anticipated to continue in spinal surgery. Patient requirements for faster, less-invasive treatments to achieve spinal fusion will continue as will the need for safely preserving motion and spinal stability in various patient populations.
Much like the field of instrumented spine surgery, PEEK continues to evolve. There also has been a growing interest in enhancing the osseoconductivity of PEEK to provide direct bone on-growth and to follow the advances made in porous metals technology to allow bone in-growth in 3-D interconnected porous PEEK structures.
Infection also is a growing area of concern with large studies reporting surgical site infection rates from 2.5 percent to 13 percent. It is expected that material modification will be needed to address this concern. Ongoing research in tissue scaffolds and antimicrobial formulations, although still in its early stages, highlight the role of PEEK biomaterials as a versatile platform for future innovation in implantable spinal devices.
References
1. http://hcupnet.ahrq.gov
2. S.G. Capps. “PEEK Cages and Spacers in Cervical Spine Fusion Applications.” Spinal News International, 4 (2007).
3. M. Boakye et al. “Anterior cervical discectomy and fusion involving a polyetheretherketone spacer and bone morphogenic protein.” J Neurosurg: Spine 2: 521-525, 2005.
4. S.E. Celik et al. “A comparison of changes over time in cervical foraminal height after tricortical iliac graft or polyetheretherketone cage placement following anterior discectomy.” J Neurosurg Spine 6: 10-16, 2007.
5. D-Y Cho et al. “Treatment of multilevel cervical fusion with cages.” Surg Neurol 62: 378-386, 2004.
6. D-Y Cho et al. “Preliminary experience using a polyetheretherketone (PEEK) cage in the treatment of cervical disc disease.” Neurosurgery 51(6): 1343-1350, 2002.
7. D-Y Cho et al. “Cage containing a biphasic calcium phosphate ceramic (Triosite) for the treatment of cervical spondylosis.” Surgical Neurology 63: 497-504, 2005.
8. S. Kahraman et al. “Polyetheretherketone (PEEK) cages for cervical interbody replacement: Clinical experience.” Turkish Neurosurgery 16(3): 120-123, 2006.
9. A.G. Kulkarni et al. “Solis cage (PEEK) for anterior cervical fusion: preliminary radiological results with emphasis on fusion and subsidence.” The Spine Journal 7: 205-209, 2007.
10. Q. Malone et al. “Surgical outcome for anterior cervical discectomy and fusion using the Signus Rabea PEEK cage.” Proceedings of the NASS 18th Annual Meeting: The Spine Journal 3: 67S-171S, 2003.
11. L Mastronardi et al. “Anterior cervical fusion with polyetheretherketone (PEEK) cages in the treatment of degenerative disc disease. Preliminary observations in 36 consecutive cases with a minimum 12-month follow-up.” Acta Neurochir 148: 307-312, 2006.
12. Z. Sekerci et al. “Early changes in the cervical foraminal area after anterior interbody fusion with polyetheretherketone (PEEK) cage containing synthetic bone particulate: a prospective study of 20 cases.” Neurological Research 28: 568-571, 2006.
13. H.M. Mayer, Minimally Invasive Spine Surgery, pp 459-465.
14. T. Brown et al. A comprehensive wear assessment of PEEK-OPTIMA for Disc Arthroplasty Applications, World Biomaterials Congress 2008, Amsterdam, Netherlands
15. T. Brown et al. “Biotribological Assessment of NuNec, a PEEK-on-PEEK Cervical Total Disc Replacement, According to ISO and ASTM Recommendations.” Spine Arthroplasty Society 2009, London, England.
16. H. Yuan et al. “Early Clinical Experience of NuNec, a Novel PEEK-On-PEEK Artificial Cervical Disc. ”Spine Arthroplasty Society 2009, London, England.
17. Grupp et al. “Alternative bearing materials for intervertebral disc arthroplasty” Biomaterials 2009 Published Online.