FDA Approves Patient-Specific UNiD Cervical Rod for Spine Surgery
FDA Approves Patient-Specific UNiD Cervical Rod for Spine Surgery
First implantation of the MEDICREA product occurred in mid-February.
Watch the video below for context on the product and associated surgery as well as its growing impact on the medical sector:
Immediately following the FDA clearance, MEDICREA successfully expanded its personalized UNiD technology and services to the cervical spine with the first implantation of the UNiD Patient-Specific Cervical rod in New York, N.Y., on Feb. 17 by Dr. Thermistocles Protopsaltis. With this latest addition, the company announced that UNiD Lab personalized spinal alignment services are now available to cover the entire cervical, thoracic and lumbar regions.
“The patient-specific cervical rod option is a natural extension of the UNiD portfolio," said Denys Sournac, MEDICREA president and CEO. "By providing patient-specific realignment implant options for the entire spine, we are again confirming MEDICREA’s leadership position in personalized, made-to-measure treatments. This continued expertise brings pioneering technologies and services to the spine that are uniquely dedicated to improving clinical results for each patient.”
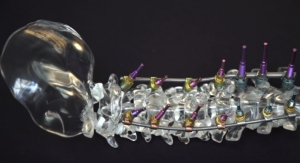
The service model for the UNiD Cervical rod follows the same effective collaboration between MEDICREA’s UNiD Lab and spinal surgeons worldwide, which has surpassed the creation of custom UNiD Thoracolumbar rods for more than 650 implantations to date. The UNiD rod is custom designed for cervical, thoracic and lumbar spine operations to scientifically match the rod’s shape with the patient’s unique spinal alignment and the surgeon’s pre-operative plan.
Following surgical intervention in the occipito-cervical spine, the shape of the rod directly impacts the fixed position of the head and neck and dramatically changes how the patient looks and feels. Surgeons who do not use UNiD are forced to manually approximate the rod shape – a challenge greatly increased for rods with two diameters, which are frequently used in this type of surgery with no existing tools to accommodate the transitional point.
This challenge is doubled as two rods are systematically used in combination – a feat requiring precision that is simply not possible with the naked eye. The UNiD Cervical rod technology removes this existing surgical barrier by creating two strictly identical single or dual-diameter rods manufactured specifically for the patient and delivered directly to the operating room.
“I am so happy to have the UNiD Cervical rod available," said Protopsaltis. "This pre-contoured patient-specific implant has eliminated the difficulty of manually bending transition rods, saving OR time and optimizing the execution of my operative plan professional collaboration with MEDICREA’s UNiD Lab translated easily into a seamless intra-operative experience and the best possible care I can offer my patient – a personalized treatment.”
UNiD Patient-Specific Cervical rods are available in two alloys (titanium TA6V ELI/cobalt chromium) and in a single-diameter (3.5mm) or dual-diameter options (3.5mm transitioning to either 5.5mm or 6.0mm). The UNiD Patient-Specific Cervical rods are secured using MEDICREA’s most-recent extension of their versatile ‘PASS’ (PolyAxial Spine System) posterior fixation platform, PASS OCT Posterior Cervical Stabilization System, which allows 11mm of gentle reduction capability across all anchorages and medial or lateral rod placement.
The PASS OCT System has been designed specifically to complement UNiD patient-specific technology, minimizing forces applied on the spine when securing the rod.
The MEDICREA Group specializes in the design, manufacture, and distribution of spinal surgery technologies. The products developed and patented by MEDICREA provide neurosurgeons and orthopedic surgeons specializing in the spine with new and less-invasive surgical solutions that are faster and easier to implement than traditional techniques. MEDICREA has become a pioneer in the manufacturing of customized implants for personalized spinal surgery with the development of a comprehensive process incorporating the software analysis of each patient, the pre-surgical planning of the surgical strategy, and the production of customized spinal osteosynthesis rods (UNiD rod) and lumbar interbody osteosynthesis cages (UNiD ALIF cage) that are made to measure by a 3D printer. The group’s headquarters are based near Lyon, France. It also has an implant and surgical instrument manufacturing facility located in La Rochelle, France, as well as four distribution subsidiaries in the United States, the United Kingdom, France and Germany.