Michael Barbella, Managing Editor02.23.17
Mike McDonald was a high school junior when the pain began.
It wasn’t too debilitating at first—barely registering, really, as discomfort. There was a sharp twinge and odd clicking sensation in his shoulder, though neither could have lasted more than a second or two. McDonald, then 17, hardly had time to react before the pangs disappeared.
Probably just a routine muscle spasm or something, McDonald thought to himself, attributing the cricks to his aggressions on the ice hockey rink.
Ah, the naiveté of youth.
McDonald couldn’t have known it then, but the twinge and “clicking sensation” he felt in his shoulder was the start of a Glenoid labrum tear—a rip in the cuff of cartilage circling the shoulder socket. Such injuries are common in certain rugged contact sports as well as those requiring repeated overhead movements (i.e., water polo, basketball, baseball, volleyball).
After graduating high school, McDonald delayed college for a year to play in the Ontario Junior Hockey League. During that time, his shoulder pain worsened and he began dislocating the injured joint on a regular basis. “This began happening every few months, partly because I’m an aggressive player,” McDonald told Northwestern Lake Forest Hospital’s in-house magazine, healthreport. “Most goalies experience lower body impact, but I move my whole body around.”
A Canadian doctor eventually diagnosed McDonald with a slight labrum tear but encouraged him to “play through it.” He did, suffering silently in the process, yet still performing well enough to be recruited by the Lake Forest College (Illinois) hockey team. The school’s grueling playing schedule, however, ultimately took a toll on McDonald’s shoulder, making it particularly susceptible to dislocation. Even so, McDonald insisted on playing through the pain—until his damaged joint slipped out of place during a game, costing his team a goal.
McDonald’s coaches had seen enough at that point. They sent the young man for a medical evaluation with team physician Roger N. Chams, M.D., a top Chicago-area orthopedic surgeon. Chams diagnosed the Foresters goalie with torn ligaments and major bone loss in his shoulder socket—two potentially career-ending injuries. Chams gave McDonald a 65 percent chance of a full recovery with traditional surgery.
“In the past, for patients with frequently dislocating shoulders—which is common among athletes—we would surgically reconstruct the shoulder or use a bone block and about 35 percent of them would fail,” Chams explained to healthreport.
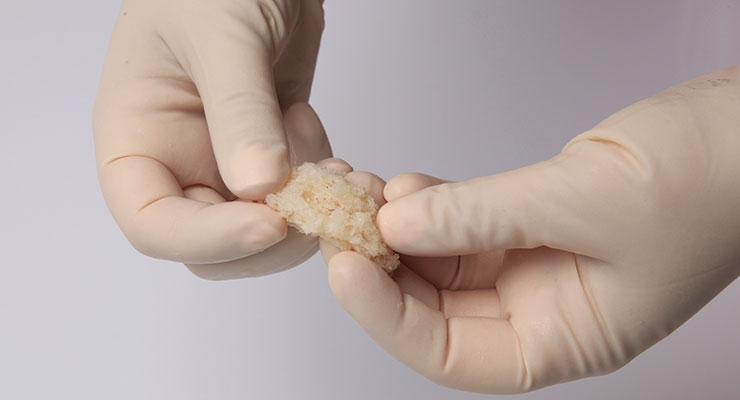
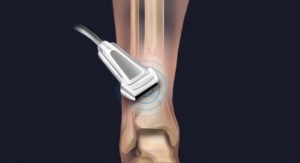
Rather than risk those odds, Chams used a new surgical technique based in human biologics to heal orthopedic conditions. Seeking to invoke Mother Nature’s mastery, Chams transplanted fresh bone and cartilage from a cadaver ankle directly into McDonald’s damaged shoulder socket in a procedure known as osteochondral allograft (OCA) reconstruction. This repair method relies upon the human body to work its magic, fusing the materials to the joint over a period of six months, during which time the patient receives physical therapy.
Summoning the body’s natural healing process is becoming a preferred treatment option in orthopedics as longer- and harder-living patients demand longer-lasting, tougher solutions to age-related conditions and athletic injuries. These remedies are also preferred for their overall improved outcomes: The OCA repair method, for example, reduces the risk of recurrent joint instability and avoids many of the complications common in traditional procedures. It also might prevent the development of arthritis, according to experts.
“The body is definitely more sophisticated than anybody realizes,” noted Alessandra Pavesio, senior vice president and chief science officer for Bioventus LLC, a Durham, N.C.-based orthobiologics developer formed in 2012 through a strategic venture between Essex Woodlands and Smith & Nephew plc.
“What has become very apparent in this field is the truly powerful repair mechanisms that the body can trigger in a physiological fashion as opposed to adding a foreign material or introducing a drug that will inevitably hit the intended pathway but will also provide an imbalance to all the other pathways that are involved,” Pavesio continued. “In orthobiologics, you really have nature’s orchestra at your disposal as compared to just a single instrument. It’s very intuitive why an approach like this is going to be safe and in the long term, more effective. The time is ripe for these technologies to become available as I believe their adoption will greatly benefit patients.”
Those benefits have long been proven, too: Clinical studies have shown that hyaluronic acid (a key component of articular cartilage) and platelet-rich plasma can reduce knee osteoarthritis pain, with the latter also effective at treating various other conditions like lateral epicondylitits, rotator cuff tears, and retro calcaneal bursitis.
Likewise, allograft and autograft tissue are both tried-and-true architects of damaged anterior cruciate ligaments, though trial data tend to support the use of autograft tissue for young, active patients under age 25 and irradiated soft-tissue allograft for older, more sedentary folks.
Surgeons choosing autograft ACL repair often use the patient’s own hamstring to rebuild the injured ligament. Autograft is considered a more natural approach to ACL reconstruction and other orthopedic procedures since it uses the patient’s own tissue, thus minimizing the risk of rejection. But this method is not without its shortcomings, as patients often experience increased postoperative pain from the second surgical (autograft) site, and potentially require longer periods of rehabilitation.
Allografts, on the other hand, eliminate the need for a second procedure, thereby reducing operating room time and expediting recovery. Yet this approach isn’t foolproof, either: Donated tissue is usually more expensive, is not always available, and is prone to biological rejection. Moreover, allografts may not incorporate themselves properly into the recipient’s own body function, and may take longer to connect with an existing joint.
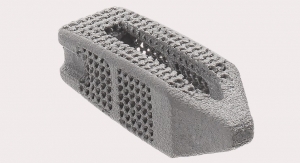
Such autograft- and allograft-related limitations are helping to fuel growth in the global orthobiologics market and spawning advancements in bone graft technology and regenerative therapies.
Among the newest innovations in bone graft material is the i-FACTOR Peptide Enhanced Bone Graft from Westminster, Colo.-based Cerapedics Inc. The graft leverages the biological activity of a synthetically derived 15-amino acid peptide (P-15) to support bone growth through cell attachment and activation, according to the company. It is approved for use as a substitute for autologous bone in anterior cervical discectomy and fusion procedures.
Northwestern University scientists, meanwhile, are on the verge of revolutionizing the future of bone grafts and reconstructive surgery with the development of “hyperelastic bone” (HB), a 3D-printed synthetic material made mostly from hydroxyapatite. Mixed with a polymer to add flexibility, HB can either be implanted under the skin to serve as a scaffold for new bone growth or used to replace lost bone.
Researchers tested the hyperelastic bone in real surgical situations, using it to fuse together two spinal vertebrae in a rat, and replace a section of unhealthy rhesus monkey skull. In both cases, the implant fully integrated with the host tissue, encouraging the growth of blood vessels and even some new bone.
Albeit promising, HB has yet to be tested on humans.
Complimenting these potentially game-changing strides in bone graft technology are harvesting and delivery systems innovations like those developed by NovaBone Products LLC, TranS1, and Stryker Corp.
NovaBone’s MacroFORM MIS Delivery System is designed for use in minimally invasive procedures, allowing surgeons to directly deliver bone marrow aspirate to a graft housed in a closed, ready-to-use cannula. The system allows physicians to precisely deliver bone grafting material to surgical sites that are not readily accessible.
TranS1’s Capital Bone Graft Harvester, on the other hand, is designed to collect reproducible autograft material from the iliac crest while its Pylon Graft Delivery tool is designed for posterolateral fixation. The Pylon deposits bone graft material through the same incision as the posterior fixation and can be used in conjunction with posterior fixation, interbody fusion, and laminectomy.
TranS1’s Capital Harvester and Pylon Delivery tool made their market debut last fall around the same time Stryker released its LITe BIO Delivery System, a tool that gives surgeons a single-handed method to deliver “any type of autograft, allograft, or synthetic bone graft material without obstructing visibility.” The patented system can be used for any type of spinal fusion procedure, including minimally invasive surgeries. Its low-profile design allows visibility through a decompression tube without obstructing the view, and disposable cannula can deliver up to 5cc of bone graft at one time.
“A significant need in the market is improving the delivery of bone graft to spinal surgery sites,” said John Mayor, vice president of global marketing for Stryker’s Spine Division. “The LITe BIO Delivery System provides tactile, visual, and audible confirmation of bone graft delivery, and the mallet-free system is designed to eliminate the impaction of bone graft.”
Indeed, delivery accuracy is as crucial to bone repair as the reconstructive technique itself. In recent years, the bone graft substitute market has been shifting away from allograft and growth factors usage to more natural remedies like those engineered by Kuros Biosciences A.G., a Swiss developer of tissue repair and regeneration products. Amidst the firm’s most advanced technologies are KUR-111 and KUR-113, both of which are designed to help heal fractures. KUR-111 consists of a natural matrix (fibrin), a targeted bone growth factor and a structural ceramic component that form a paste for direct insertion into fracture voids. The material can be left to polymerize in situ to adopt the defect’s shape and form an ideal space-filling graft substitute that resists compression while providing optimal biological support for healing.
Kuros has completed a Phase IIb clinical trial assessing KUR-111’s potential to treat tibial plateau fractures requiring fixation and grafting. Tibial plateau fractures are often complex and usually caused by high-energy trauma such as automobile or industrial accidents, or sports injuries.
KUR-113 is also applied directly into fracture voids, but forms a gel-like material that infiltrates fracture sites without disturbing the surrounding tissue. Like its sister product, this matrix aims to improve fracture healing.
“The regenerative mechanisms of the body are extremely complex,” Pavesio said. “To date, the standard of care in just about any [orthopedic] surgery remains an autologous graft because it is very, very hard to synthetically reproduce a tissue, considering all the complexity that goes into it. That’s why allografts are so widely used and why they have clinical merit. We’re not reproducing the complexity involved or the shared pathways, we’re just implanting what Mother Nature has already done. When you’re trying to duplicate something, you’re simplifying it to the point where you risk changing its subleties. It’s extremely complicated to duplicate what we have. I think our best bet is to find ways to identify therapeutic strategies that will trigger the intrinsic self-repair mechanism of the tissue for example, so we can utilize orthobiologic approaches to attract resident cells to injured tissue and do the work. What we can do is provide a substrate for these cells to work as well as a signal for the proper molecules to come and do their magic.’ ”
One of the most gifted famuli seems to be stem cells, primitive organisms with an amazing capacity for self-renewal and specialization. These cells play a leading role in the body’s healing process and have been used for decades to treat various conditions including leukemia, sickle cell anemia, lymphoma, and other blood diseases.
Stem cells have more recently been used in the orthopedic industry to treat arthritis, bone fractures and non-unions, degenerative vertebral discs, damaged cartilage, and torn tendons/ligaments. Most procedures are still experimental, according to experts, but a growing number of therapies are being offered by clinics touting clinically unproven results. Concerns over the spread of these untested treatments has prompted the U.S. Food and Drug Administration to re-examine its guidelines on human-derived therapies (see sidebar).
The adult stem cells considered the most propitious for orthopedic applications are mesenchymal cells, which have been isolated from various tissue sources including bone marrow, adipose tissue, periosteum, and the synovial lining.
“By taking advantage of new scientific insights on how the body repairs, regenerates, and heals tissue that results from trauma or age, new therapies are being pioneered that focus on the repair of joints versus replacement of joints,” noted Andrew McGillicuddy, CEO of Endocellutions Inc., a Marshfield, Mass.-based developer of regenerative medicine technology. “At the forefront of these initiatives is the use of stem cells from bone marrow. The natural response to any damaged tissue is to mobilize stem cells from the marrow space to the defect site. By sourcing marrow and transplanting it to the area of trauma, the clinician is exactly mimicking and supplementing the body’s natural response to injury.”
Endocellutions’ mimicry of Mother Nature is channeled through a bone marrow device called MarrowCellution. The tool enables clinicians to aspirate several small volumes of concentrated marrow across a broad geography of marrow space, the company claims.
MarrowCellution precisely repositions the aspiration system to a new location in the marrow after each mL collected, but never uses more than one entry point. The product is designed to draw marrow from side ports while avoiding dilution with the peripheral blood that often pools below the tip as the aspiration needle is withdrawn.
The proprietary device optimizes stem cell yields by minimizing peripheral blood contamination. “Using this technology, with a simple marrow aspiration of 10 mL, a clinician is able to get 300 percent more stem cells than if 60 mL was drawn and concentrated by a centrifuge down to 10 mL,” McGillicuddy explained. “Stem cells are what heal tissue.”
Stryker’s tissue-healing technology also involves the use of mesenchymal stem cells. Its BIO4 Viable Bone Matrix, developed by Osiris Therapeutics Inc. (the companies partnered in late 2014 to expand Stryker’s biologics portfolio), is a viable bone matrix containing endogenous bone-forming cells designed to facilitate bone repair and regeneration. It aims to provide a safe alternative to bone autograft and minimize the potential for harvest site co-morbidities.
“Stryker’s BIO4 Viable Bone Matrix retains viable cells and retains all native bone activities involved in bone repair and regeneration: osteoconductive, osteoinductive, osteogenic, and angiogenic,” Mayor said. “The trend away from growth factors is opening opportunities to meet surgeons’ needs and preferences with a variety of other options. These technologies include non- proprietary allograft chips, synthetics, demineralized bone matrix, and stem cells. Each has a different function or mechanism of action. Some act primarily as scaffolds, as in the case of allograft chips and synthetics, and others have signals that stimulate bone growth in the body, such as demineralized bone matrix and stem cells. The market is looking for alternatives that provide an active environment to stimulate bone growth in a safe way.”
It wasn’t too debilitating at first—barely registering, really, as discomfort. There was a sharp twinge and odd clicking sensation in his shoulder, though neither could have lasted more than a second or two. McDonald, then 17, hardly had time to react before the pangs disappeared.
Probably just a routine muscle spasm or something, McDonald thought to himself, attributing the cricks to his aggressions on the ice hockey rink.
Ah, the naiveté of youth.
McDonald couldn’t have known it then, but the twinge and “clicking sensation” he felt in his shoulder was the start of a Glenoid labrum tear—a rip in the cuff of cartilage circling the shoulder socket. Such injuries are common in certain rugged contact sports as well as those requiring repeated overhead movements (i.e., water polo, basketball, baseball, volleyball).
After graduating high school, McDonald delayed college for a year to play in the Ontario Junior Hockey League. During that time, his shoulder pain worsened and he began dislocating the injured joint on a regular basis. “This began happening every few months, partly because I’m an aggressive player,” McDonald told Northwestern Lake Forest Hospital’s in-house magazine, healthreport. “Most goalies experience lower body impact, but I move my whole body around.”
A Canadian doctor eventually diagnosed McDonald with a slight labrum tear but encouraged him to “play through it.” He did, suffering silently in the process, yet still performing well enough to be recruited by the Lake Forest College (Illinois) hockey team. The school’s grueling playing schedule, however, ultimately took a toll on McDonald’s shoulder, making it particularly susceptible to dislocation. Even so, McDonald insisted on playing through the pain—until his damaged joint slipped out of place during a game, costing his team a goal.
McDonald’s coaches had seen enough at that point. They sent the young man for a medical evaluation with team physician Roger N. Chams, M.D., a top Chicago-area orthopedic surgeon. Chams diagnosed the Foresters goalie with torn ligaments and major bone loss in his shoulder socket—two potentially career-ending injuries. Chams gave McDonald a 65 percent chance of a full recovery with traditional surgery.
“In the past, for patients with frequently dislocating shoulders—which is common among athletes—we would surgically reconstruct the shoulder or use a bone block and about 35 percent of them would fail,” Chams explained to healthreport.
Rather than risk those odds, Chams used a new surgical technique based in human biologics to heal orthopedic conditions. Seeking to invoke Mother Nature’s mastery, Chams transplanted fresh bone and cartilage from a cadaver ankle directly into McDonald’s damaged shoulder socket in a procedure known as osteochondral allograft (OCA) reconstruction. This repair method relies upon the human body to work its magic, fusing the materials to the joint over a period of six months, during which time the patient receives physical therapy.
Summoning the body’s natural healing process is becoming a preferred treatment option in orthopedics as longer- and harder-living patients demand longer-lasting, tougher solutions to age-related conditions and athletic injuries. These remedies are also preferred for their overall improved outcomes: The OCA repair method, for example, reduces the risk of recurrent joint instability and avoids many of the complications common in traditional procedures. It also might prevent the development of arthritis, according to experts.
“The body is definitely more sophisticated than anybody realizes,” noted Alessandra Pavesio, senior vice president and chief science officer for Bioventus LLC, a Durham, N.C.-based orthobiologics developer formed in 2012 through a strategic venture between Essex Woodlands and Smith & Nephew plc.
“What has become very apparent in this field is the truly powerful repair mechanisms that the body can trigger in a physiological fashion as opposed to adding a foreign material or introducing a drug that will inevitably hit the intended pathway but will also provide an imbalance to all the other pathways that are involved,” Pavesio continued. “In orthobiologics, you really have nature’s orchestra at your disposal as compared to just a single instrument. It’s very intuitive why an approach like this is going to be safe and in the long term, more effective. The time is ripe for these technologies to become available as I believe their adoption will greatly benefit patients.”
Those benefits have long been proven, too: Clinical studies have shown that hyaluronic acid (a key component of articular cartilage) and platelet-rich plasma can reduce knee osteoarthritis pain, with the latter also effective at treating various other conditions like lateral epicondylitits, rotator cuff tears, and retro calcaneal bursitis.
Likewise, allograft and autograft tissue are both tried-and-true architects of damaged anterior cruciate ligaments, though trial data tend to support the use of autograft tissue for young, active patients under age 25 and irradiated soft-tissue allograft for older, more sedentary folks.
Surgeons choosing autograft ACL repair often use the patient’s own hamstring to rebuild the injured ligament. Autograft is considered a more natural approach to ACL reconstruction and other orthopedic procedures since it uses the patient’s own tissue, thus minimizing the risk of rejection. But this method is not without its shortcomings, as patients often experience increased postoperative pain from the second surgical (autograft) site, and potentially require longer periods of rehabilitation.
Allografts, on the other hand, eliminate the need for a second procedure, thereby reducing operating room time and expediting recovery. Yet this approach isn’t foolproof, either: Donated tissue is usually more expensive, is not always available, and is prone to biological rejection. Moreover, allografts may not incorporate themselves properly into the recipient’s own body function, and may take longer to connect with an existing joint.
Such autograft- and allograft-related limitations are helping to fuel growth in the global orthobiologics market and spawning advancements in bone graft technology and regenerative therapies.
Among the newest innovations in bone graft material is the i-FACTOR Peptide Enhanced Bone Graft from Westminster, Colo.-based Cerapedics Inc. The graft leverages the biological activity of a synthetically derived 15-amino acid peptide (P-15) to support bone growth through cell attachment and activation, according to the company. It is approved for use as a substitute for autologous bone in anterior cervical discectomy and fusion procedures.
Northwestern University scientists, meanwhile, are on the verge of revolutionizing the future of bone grafts and reconstructive surgery with the development of “hyperelastic bone” (HB), a 3D-printed synthetic material made mostly from hydroxyapatite. Mixed with a polymer to add flexibility, HB can either be implanted under the skin to serve as a scaffold for new bone growth or used to replace lost bone.
Researchers tested the hyperelastic bone in real surgical situations, using it to fuse together two spinal vertebrae in a rat, and replace a section of unhealthy rhesus monkey skull. In both cases, the implant fully integrated with the host tissue, encouraging the growth of blood vessels and even some new bone.
Albeit promising, HB has yet to be tested on humans.
Complimenting these potentially game-changing strides in bone graft technology are harvesting and delivery systems innovations like those developed by NovaBone Products LLC, TranS1, and Stryker Corp.
NovaBone’s MacroFORM MIS Delivery System is designed for use in minimally invasive procedures, allowing surgeons to directly deliver bone marrow aspirate to a graft housed in a closed, ready-to-use cannula. The system allows physicians to precisely deliver bone grafting material to surgical sites that are not readily accessible.
TranS1’s Capital Bone Graft Harvester, on the other hand, is designed to collect reproducible autograft material from the iliac crest while its Pylon Graft Delivery tool is designed for posterolateral fixation. The Pylon deposits bone graft material through the same incision as the posterior fixation and can be used in conjunction with posterior fixation, interbody fusion, and laminectomy.
TranS1’s Capital Harvester and Pylon Delivery tool made their market debut last fall around the same time Stryker released its LITe BIO Delivery System, a tool that gives surgeons a single-handed method to deliver “any type of autograft, allograft, or synthetic bone graft material without obstructing visibility.” The patented system can be used for any type of spinal fusion procedure, including minimally invasive surgeries. Its low-profile design allows visibility through a decompression tube without obstructing the view, and disposable cannula can deliver up to 5cc of bone graft at one time.
“A significant need in the market is improving the delivery of bone graft to spinal surgery sites,” said John Mayor, vice president of global marketing for Stryker’s Spine Division. “The LITe BIO Delivery System provides tactile, visual, and audible confirmation of bone graft delivery, and the mallet-free system is designed to eliminate the impaction of bone graft.”
Indeed, delivery accuracy is as crucial to bone repair as the reconstructive technique itself. In recent years, the bone graft substitute market has been shifting away from allograft and growth factors usage to more natural remedies like those engineered by Kuros Biosciences A.G., a Swiss developer of tissue repair and regeneration products. Amidst the firm’s most advanced technologies are KUR-111 and KUR-113, both of which are designed to help heal fractures. KUR-111 consists of a natural matrix (fibrin), a targeted bone growth factor and a structural ceramic component that form a paste for direct insertion into fracture voids. The material can be left to polymerize in situ to adopt the defect’s shape and form an ideal space-filling graft substitute that resists compression while providing optimal biological support for healing.
Kuros has completed a Phase IIb clinical trial assessing KUR-111’s potential to treat tibial plateau fractures requiring fixation and grafting. Tibial plateau fractures are often complex and usually caused by high-energy trauma such as automobile or industrial accidents, or sports injuries.
KUR-113 is also applied directly into fracture voids, but forms a gel-like material that infiltrates fracture sites without disturbing the surrounding tissue. Like its sister product, this matrix aims to improve fracture healing.
“The regenerative mechanisms of the body are extremely complex,” Pavesio said. “To date, the standard of care in just about any [orthopedic] surgery remains an autologous graft because it is very, very hard to synthetically reproduce a tissue, considering all the complexity that goes into it. That’s why allografts are so widely used and why they have clinical merit. We’re not reproducing the complexity involved or the shared pathways, we’re just implanting what Mother Nature has already done. When you’re trying to duplicate something, you’re simplifying it to the point where you risk changing its subleties. It’s extremely complicated to duplicate what we have. I think our best bet is to find ways to identify therapeutic strategies that will trigger the intrinsic self-repair mechanism of the tissue for example, so we can utilize orthobiologic approaches to attract resident cells to injured tissue and do the work. What we can do is provide a substrate for these cells to work as well as a signal for the proper molecules to come and do their magic.’ ”
One of the most gifted famuli seems to be stem cells, primitive organisms with an amazing capacity for self-renewal and specialization. These cells play a leading role in the body’s healing process and have been used for decades to treat various conditions including leukemia, sickle cell anemia, lymphoma, and other blood diseases.
Stem cells have more recently been used in the orthopedic industry to treat arthritis, bone fractures and non-unions, degenerative vertebral discs, damaged cartilage, and torn tendons/ligaments. Most procedures are still experimental, according to experts, but a growing number of therapies are being offered by clinics touting clinically unproven results. Concerns over the spread of these untested treatments has prompted the U.S. Food and Drug Administration to re-examine its guidelines on human-derived therapies (see sidebar).
The adult stem cells considered the most propitious for orthopedic applications are mesenchymal cells, which have been isolated from various tissue sources including bone marrow, adipose tissue, periosteum, and the synovial lining.
“By taking advantage of new scientific insights on how the body repairs, regenerates, and heals tissue that results from trauma or age, new therapies are being pioneered that focus on the repair of joints versus replacement of joints,” noted Andrew McGillicuddy, CEO of Endocellutions Inc., a Marshfield, Mass.-based developer of regenerative medicine technology. “At the forefront of these initiatives is the use of stem cells from bone marrow. The natural response to any damaged tissue is to mobilize stem cells from the marrow space to the defect site. By sourcing marrow and transplanting it to the area of trauma, the clinician is exactly mimicking and supplementing the body’s natural response to injury.”
Endocellutions’ mimicry of Mother Nature is channeled through a bone marrow device called MarrowCellution. The tool enables clinicians to aspirate several small volumes of concentrated marrow across a broad geography of marrow space, the company claims.
MarrowCellution precisely repositions the aspiration system to a new location in the marrow after each mL collected, but never uses more than one entry point. The product is designed to draw marrow from side ports while avoiding dilution with the peripheral blood that often pools below the tip as the aspiration needle is withdrawn.
The proprietary device optimizes stem cell yields by minimizing peripheral blood contamination. “Using this technology, with a simple marrow aspiration of 10 mL, a clinician is able to get 300 percent more stem cells than if 60 mL was drawn and concentrated by a centrifuge down to 10 mL,” McGillicuddy explained. “Stem cells are what heal tissue.”
Stryker’s tissue-healing technology also involves the use of mesenchymal stem cells. Its BIO4 Viable Bone Matrix, developed by Osiris Therapeutics Inc. (the companies partnered in late 2014 to expand Stryker’s biologics portfolio), is a viable bone matrix containing endogenous bone-forming cells designed to facilitate bone repair and regeneration. It aims to provide a safe alternative to bone autograft and minimize the potential for harvest site co-morbidities.
“Stryker’s BIO4 Viable Bone Matrix retains viable cells and retains all native bone activities involved in bone repair and regeneration: osteoconductive, osteoinductive, osteogenic, and angiogenic,” Mayor said. “The trend away from growth factors is opening opportunities to meet surgeons’ needs and preferences with a variety of other options. These technologies include non- proprietary allograft chips, synthetics, demineralized bone matrix, and stem cells. Each has a different function or mechanism of action. Some act primarily as scaffolds, as in the case of allograft chips and synthetics, and others have signals that stimulate bone growth in the body, such as demineralized bone matrix and stem cells. The market is looking for alternatives that provide an active environment to stimulate bone growth in a safe way.”
|
All they want is control of their destinies. Nothing more, nothing less. In truth, Georgianna Crocker and Ted Gradel are fighting for the right to medically use their own stem cells without government interference. “When I look at that little vial, the little sliver of SVF—stromal vascular fraction—that is sitting at the bottom of that test tube, those came out of my body and those are my cells,” Gradel told a U.S. Food and Drug Administration (FDA) panel last fall. “I don’t really care...if they have been manipulated either minimally or maximally, I feel like I should have the right to have those cells injected back into my own body...That decision should rest between me and my physician.” Perhaps it should, but concerns over the growing number of U.S. clinics dispensing untested—and in many cases, unproven—stem cell treatment has prompted the FDA to rethink its rules on human tissue-derived therapies. The agency held a two-day hearing last September on new guidance that clarifies existing regulations on stem cell remedies. Heralded for their ability to multiply and morph into any type of biological cell, stem cells have been used for decades (with the FDA’s blessing) to treat certain blood disorders and leukemia. Their use has become more widespread in recent years with endorsements from high-profile athletes like Bartolo Colon and Peyton Manning, both of whom used stem cell therapies to heal bulging discs, damaged ligaments, and a torn rotator cuff. Nevertheless, stem cell science is still evolving and proving difficult to understand, as the cells themselves can sometimes be unmanageable and harmful to living organisms. FDA approval is currently not required for stem cell therapies as long as the recruited cells are not significantly altered, are used in a similar way to their original purpose, and are derived from a patient’s own stem cell bank. The new guidelines, however, add a few more stipulations for sidestepping regulators: Stem cell function must be identical in both the donor and recipient; treatment cells cannot affect the recipient’s entire body; and manufacturers can only manipulate the cells “minimally.” The revised rules also specify the chemicals manufacturers can use to treat cells and prevent disease transmission. FDA officials insist the revamped guidelines are meant to clarify existing regulations, but stem cell advocates fret the new rules will deny patients a viable treatment alternative for debilitating illnesses. For Crocker, the agency’s “hands-off” approach to stem cell regulation has been a blessing—the fat cells harvested from her body two years ago virtually eradicated the rheumatoid arthritis that had ravaged her joints for nearly a decade. During last fall’s hearing, she recounted her journey from arthritis victim to victor, touting her negligible C-reactive protein (inflammation marker) levels and normal white blood cell counts. The turnaround in Crocker’s health has been so dramatic, in fact, that she ran a half-marathon last July. “...I ask that you strengthen my rights as a patient to be treated with my own stem cells and to accelerate this availability of treatment that is safe and effective, and to please not classify my own cells as a drug,” Crocker appealed to FDA officials. “They are my own cells and I ask that you respectfully treat them that way.” The FDA’s intention, of course, is not one of disrespect but rather safety. Agency administrators object to testaments like Crocker’s that proclaim stem cell therapy “safe and effective,” since its safety and efficacy are still largely unproven. Despite promising preliminary evidence, scientific studies thus far “have not reliably demonstrated the effectiveness of stem cell treatments even in some of the most systematically studied conditions such as heart failure and graft-versus-host disease,” FDA bigwigs wrote in a New England Journal of Medicine article last November. This lack of evidence is worrisome.” Even more troubling to FDA executives is the proliferation of unregulated stem cell treatments by hundreds of U.S. clinics that lack the scientific evidence to support their work. Doctors, researchers, and nurses at the FDA’s panel hearing implored the agency to increase its oversight to better protect patients and control an industry that some experts have likened to the Wild West. “The out-of-control marketplace for stem cell interventions needs effective regulatory oversight,” Leigh Turner, an associate professor at the University of Minnesota Center for Bioethics (Minneapolis), declared to the FDA’s Biologics Evaluation and Research Center panel. “I therefore hope the draft guidances are more than stage props and this hearing is more than public theater. When patient safety and public health are at stake, the FDA must do more than function as a paper tiger. It is time for action.” — M.B. |