PR Newswire03.16.17
DePuy Synthes Mitek Sports Medicine has launched a new suite of knee arthroscopy solutions to help improve operating room efficiency, simplify ACL and meniscus repair, and make these surgeries more reproducible. The new platform expands the company's leadership in products and instruments for knee arthroscopy by combining performance with an easy-to-use solution for every step of the procedure. The announcement was made here at the American Academy of Orthopaedic Surgeons Annual Meeting.
Meniscus and ACL surgery are two of the most common knee procedures performed worldwide. In 2016 580,000 meniscal repairs and more than 865,000 ACL surgeries were projected to be performed worldwide.1 With such a high-volume of surgeries, creating efficiency in the operating room through simple, versatile instruments and implants is critical for surgeons.
The new suite of products from Mitek Sports Medicine is designed to promote operating room efficiency and simplicity from the start to finish of the procedure, together with implants that may help promote stability:
"The new products added to the Mitek Sports Medicine knee platform are well designed," said Amir R. Moinfar, MD, orthopedic surgeon at Elite Orthopaedic and Musculoskeletal Center in Glen Burnie, Md. "Either used individually or in concert with one another, their numerous features afford the opportunity to perform ACL reconstruction and meniscus repair safely, reproducibly and efficiently, ultimately assisting me in helping my patients."
Matt Jewett, platform leader, Mitek Sports Medicine said, "We recognize that our customers need solutions for knee arthroscopy that deliver performance, reproducibility and operating room efficiency. We are very excited to be bringing these innovative knee products to our customers to address these needs at every step and help them treat patients with ACL and meniscal injuries."
References
1Millennium Research Group Sports Medicine Device Market 2015 Analyses: US, EMEA, ASPAC and LATAM Markets, September 2016.
2DePuy Synthes Mitek Sports Medicine, Raynham, MA. Document 103304691 2016.
Meniscus and ACL surgery are two of the most common knee procedures performed worldwide. In 2016 580,000 meniscal repairs and more than 865,000 ACL surgeries were projected to be performed worldwide.1 With such a high-volume of surgeries, creating efficiency in the operating room through simple, versatile instruments and implants is critical for surgeons.
The new suite of products from Mitek Sports Medicine is designed to promote operating room efficiency and simplicity from the start to finish of the procedure, together with implants that may help promote stability:
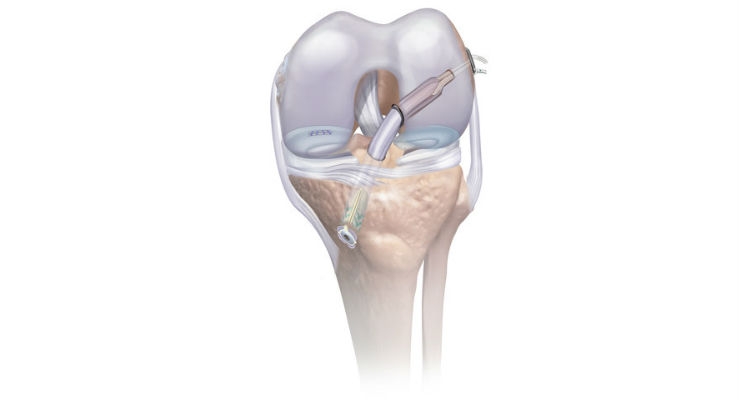
- The SPEEDTRAP Graft Prep System allows the surgeon to easily and quickly whipstitch, or suture, one tendon end without using a needle in about 20 seconds, which is at least 77 percent faster2 than traditional techniques and offers strong tension.
- The TRUESPAN Meniscal Repair System streamlines arthroscopic meniscal repair by offering a unique delivery system with an ergonomic handle, single trigger, and auto-reloading mechanism for quick, simple one-handed use. The TRUESPAN System also features the only 24-degree curved needle option on the market, which may provide better access to challenging tear locations.
- The TWIST Retrograde Reamer is used to drill the tunnels for graft placement during ACL reconstruction. The TWISTR Reamer can be set to drill 13 different tunnel diameters making it the only one-size-fits-all device of its kind on the market. The adjustable design simplifies inventory management by eliminating the need to stock multiple reamer sizes and help reduce cost during cases where more than one size reamer is needed. This product will be commercially available in the US in Q3 2017.
- The RIGIDLOOP Adjustable Cortical System, a titanium cortical button and adjustable loop implant, holds the graft in place in the femoral tunnel. The innovative design with adjustable loops eliminates need for multiple size implants. Simple one-handed tensioning technique allows surgeons to advance graft to completely fill the socket with stronger fixation and less graft displacement compared to similar adjustable loop devices.
- The INTRAFIX ADVANCE Tibial Fastener System provides rigid fixation of the graft in the tibial tunnel. The sheath and screw implant duo is designed to protect soft tissue grafts and promote integration with the surrounding bone to achieve a strong and stable fixation.
"The new products added to the Mitek Sports Medicine knee platform are well designed," said Amir R. Moinfar, MD, orthopedic surgeon at Elite Orthopaedic and Musculoskeletal Center in Glen Burnie, Md. "Either used individually or in concert with one another, their numerous features afford the opportunity to perform ACL reconstruction and meniscus repair safely, reproducibly and efficiently, ultimately assisting me in helping my patients."
Matt Jewett, platform leader, Mitek Sports Medicine said, "We recognize that our customers need solutions for knee arthroscopy that deliver performance, reproducibility and operating room efficiency. We are very excited to be bringing these innovative knee products to our customers to address these needs at every step and help them treat patients with ACL and meniscal injuries."
References
1Millennium Research Group Sports Medicine Device Market 2015 Analyses: US, EMEA, ASPAC and LATAM Markets, September 2016.
2DePuy Synthes Mitek Sports Medicine, Raynham, MA. Document 103304691 2016.