Michigan Medicine - University of Michigan03.23.17
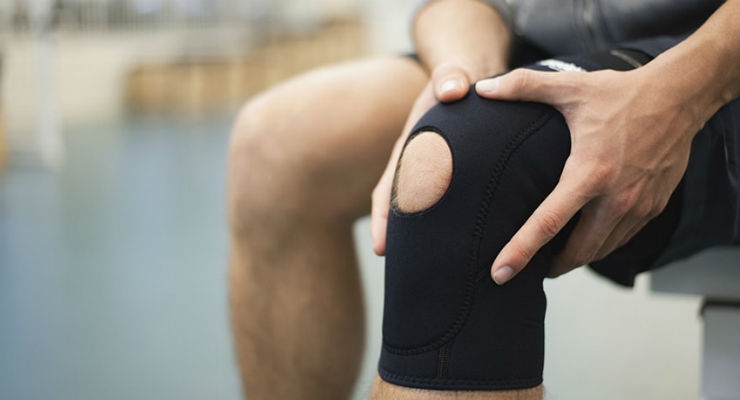
Anterior cruciate ligament injuries are devastating for athletes of any age.
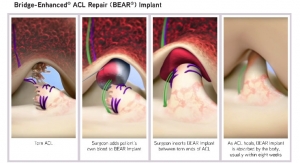
Typically, orthopedic surgeons can get athletes back to their sport with ACL reconstruction surgery. The arthroscopic procedure restores stability in the knee by replacing the torn ligament with a tendon graft.
But what happens when the reconstruction surgery isn't successful?
At the American Academy of Orthopaedic Surgeons Annual Meeting last week in San Diego, Asheesh Bedi, M.D., service chief and Gehring Professor of Orthopaedic Surgery at the University of Michigan Comprehensive Musculoskeletal Center, presented on the topic and next steps that surgeons can take when treating ACL tears.

Asheesh Bedi, M.D. (Credit: Michigan Medicine)
Bedi is the head orthopedic team physician for the University of Michigan Athletic Program, a team physician for the Detroit Lions of the National Football League and the head orthopedic consultant for the National Basketball Players Association.
Bedi spoke more about the issue and his latest research on the surgery.
What types of patients need an ACL reconstruction?
Bedi: Any patient who is active with cutting and pivoting sports. Frequently, young and active athletes are affected.
You discussed during your session that the reconstruction can fail. How so?
Bedi: This means that despite a well-performed surgery, the ligament re-tears. This is unfortunate, as it requires a repeat or "revision" surgery, and the associated recovery, which can affect multiple athletic seasons. The outcomes are generally less favorable with repeat surgery as well.
What are some correctable factors surgeons can take to help these patients?
Bedi: This is the focus of active research. Can we do a better operation and recognize missed factors that may increase the risk more in some patients than others? These may be anatomic or biologic factors that can increase risk, and perhaps if we addressed them in addition to the ACL reconstruction, may reduce failure rates. Young and hypermobile female athletes may be at the highest risk.
Are you seeing any trends in ACL reconstruction?
Bedi: Surgeries have evolved considerably with improved technology and our understanding to more accurately recreate the anatomy of the ACL. That being said, we still face the challenges of tendon healing to bone, and improved technology may still not overcome the challenging environment for biologic healing.
You also discussed your current research during the session. Could you share some highlights?
Bedi: We are looking at those additional risk factors, both anatomic and biologic. We are looking at ways to improve muscle recovery after ACL surgery and modifications at the time of surgery that may help to protect our ACL reconstruction.
Our work suggests that a favorable outcome may depend not just on a well-done surgery, but on recognizing other, often occult issues, that may predispose some athletes to greater risk than others. A more comprehensive approach that is biological and technical may be the key in the 21st century to lower failure rates and an expedited recovery and return to sport.
Typically, orthopedic surgeons can get athletes back to their sport with ACL reconstruction surgery. The arthroscopic procedure restores stability in the knee by replacing the torn ligament with a tendon graft.
But what happens when the reconstruction surgery isn't successful?
At the American Academy of Orthopaedic Surgeons Annual Meeting last week in San Diego, Asheesh Bedi, M.D., service chief and Gehring Professor of Orthopaedic Surgery at the University of Michigan Comprehensive Musculoskeletal Center, presented on the topic and next steps that surgeons can take when treating ACL tears.

Asheesh Bedi, M.D. (Credit: Michigan Medicine)
Bedi spoke more about the issue and his latest research on the surgery.
What types of patients need an ACL reconstruction?
Bedi: Any patient who is active with cutting and pivoting sports. Frequently, young and active athletes are affected.
You discussed during your session that the reconstruction can fail. How so?
Bedi: This means that despite a well-performed surgery, the ligament re-tears. This is unfortunate, as it requires a repeat or "revision" surgery, and the associated recovery, which can affect multiple athletic seasons. The outcomes are generally less favorable with repeat surgery as well.
What are some correctable factors surgeons can take to help these patients?
Bedi: This is the focus of active research. Can we do a better operation and recognize missed factors that may increase the risk more in some patients than others? These may be anatomic or biologic factors that can increase risk, and perhaps if we addressed them in addition to the ACL reconstruction, may reduce failure rates. Young and hypermobile female athletes may be at the highest risk.
Are you seeing any trends in ACL reconstruction?
Bedi: Surgeries have evolved considerably with improved technology and our understanding to more accurately recreate the anatomy of the ACL. That being said, we still face the challenges of tendon healing to bone, and improved technology may still not overcome the challenging environment for biologic healing.
You also discussed your current research during the session. Could you share some highlights?
Bedi: We are looking at those additional risk factors, both anatomic and biologic. We are looking at ways to improve muscle recovery after ACL surgery and modifications at the time of surgery that may help to protect our ACL reconstruction.
Our work suggests that a favorable outcome may depend not just on a well-done surgery, but on recognizing other, often occult issues, that may predispose some athletes to greater risk than others. A more comprehensive approach that is biological and technical may be the key in the 21st century to lower failure rates and an expedited recovery and return to sport.