Business Wire10.10.17
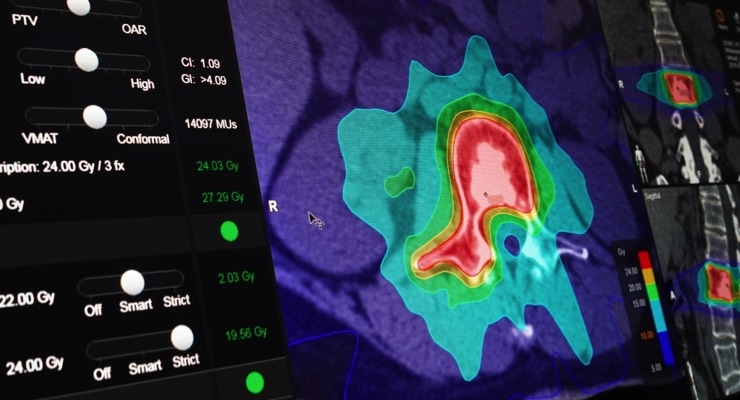
Brainlab has received U.S. Food and Drug Administration (FDA) clearance for Elements Spine SRS and Elements Cranial SRS, two software applications that aid in the patient-tailored planning of radiosurgery treatments for indications of the spine and brain. With FDA clearance of these software modules, the Brainlab Elements portfolio of applications that facilitate quick planning, spare organs at risk, and create highly conformal radiation doses in the brain and spine is complete and ready for clinical use in the United States.
“It became clear to us many years ago that radiosurgery plans must become more tailored to both the indication and the specific patient being treated,” commented Stefan Vilsmeier, president and CEO of Brainlab. “With the positive reception of our Elements Multiple Brain Mets SRS software, which has already been used to create plans for hundreds of patients around the globe, Elements Cranial SRS and Spine SRS were a natural progression toward that goal.”
Brainlab Elements Cranial SRS can create radiosurgery plans in less than 15 minutes from start to finish. The software supports plans for numerous cranial indications, including arteriovenous malformations (AVM), pituitary adenoma, vestibular schwannoma, glioma, meningioma and large brain metastases. A patented integrated 4π algorithm that optimizes beam trajectories, automatically spares surrounding healthy tissue and organs at risk with monitor unit-efficient delivery.
Historically, radiosurgery has been used with caution for spine tumors due to the risk of causing damage to the delicate spinal cord. Brainlab Elements Spine SRS addresses this issue and other challenges facing spine radiosurgery treatments, such as accounting for variations in the curvature of the spine and ensuring that high doses of radiation are delivered to the tumor and not the spinal cord itself. Contouring is streamlined and incorporates guidelines set by the International Spine Consortium.
After gaining CE approval in May 2017, Elements Spine SRS and Cranial SRS were successfully introduced into clinical use worldwide. The first patients were treated with Spine SRS at Instituto Privado de Radioterapia Oncologica S.A. in Cordoba, Argentina, and with Spine SRS and Cranial SRS at University Hospital Hamburg-Eppendorf in Germany.
“We are highly impressed with the dosimetric results and the speed of plan optimization,” said Manuel Todorovic, head of clinical physics at University Hospital Hamburg-Eppendorf. “We ran a few plan comparisons between our other planning software and Elements Cranial SRS. The Brainlab software either produced plans of higher quality or plans of comparable quality in significantly shorter time.”
Brainlab develops, manufactures and markets software-driven medical technology, enabling access to advanced, less invasive patient treatments. Brainlab technology powers treatments in radiosurgery as well as numerous surgical fields including neurosurgery, orthopedic, ENT, CMF, spine and trauma. Founded in Munich in 1989, Brainlab has over 11,800 systems installed in more than 100 countries.
“It became clear to us many years ago that radiosurgery plans must become more tailored to both the indication and the specific patient being treated,” commented Stefan Vilsmeier, president and CEO of Brainlab. “With the positive reception of our Elements Multiple Brain Mets SRS software, which has already been used to create plans for hundreds of patients around the globe, Elements Cranial SRS and Spine SRS were a natural progression toward that goal.”
Brainlab Elements Cranial SRS can create radiosurgery plans in less than 15 minutes from start to finish. The software supports plans for numerous cranial indications, including arteriovenous malformations (AVM), pituitary adenoma, vestibular schwannoma, glioma, meningioma and large brain metastases. A patented integrated 4π algorithm that optimizes beam trajectories, automatically spares surrounding healthy tissue and organs at risk with monitor unit-efficient delivery.
Historically, radiosurgery has been used with caution for spine tumors due to the risk of causing damage to the delicate spinal cord. Brainlab Elements Spine SRS addresses this issue and other challenges facing spine radiosurgery treatments, such as accounting for variations in the curvature of the spine and ensuring that high doses of radiation are delivered to the tumor and not the spinal cord itself. Contouring is streamlined and incorporates guidelines set by the International Spine Consortium.
After gaining CE approval in May 2017, Elements Spine SRS and Cranial SRS were successfully introduced into clinical use worldwide. The first patients were treated with Spine SRS at Instituto Privado de Radioterapia Oncologica S.A. in Cordoba, Argentina, and with Spine SRS and Cranial SRS at University Hospital Hamburg-Eppendorf in Germany.
“We are highly impressed with the dosimetric results and the speed of plan optimization,” said Manuel Todorovic, head of clinical physics at University Hospital Hamburg-Eppendorf. “We ran a few plan comparisons between our other planning software and Elements Cranial SRS. The Brainlab software either produced plans of higher quality or plans of comparable quality in significantly shorter time.”
Brainlab develops, manufactures and markets software-driven medical technology, enabling access to advanced, less invasive patient treatments. Brainlab technology powers treatments in radiosurgery as well as numerous surgical fields including neurosurgery, orthopedic, ENT, CMF, spine and trauma. Founded in Munich in 1989, Brainlab has over 11,800 systems installed in more than 100 countries.