PR Newswire10.12.17
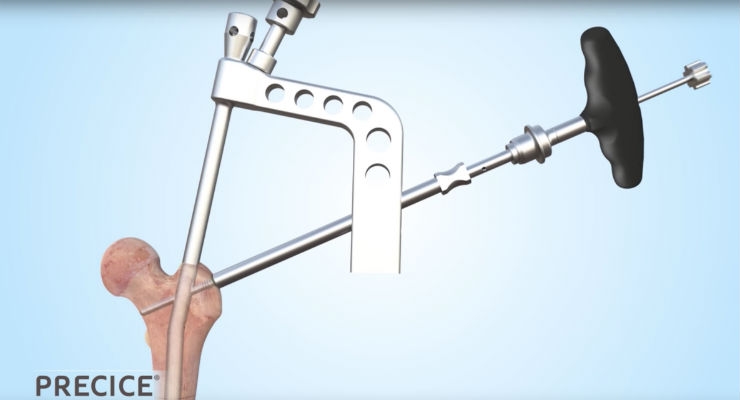
NuVasive Inc., a medical device company focused on transforming spine surgery with minimally disruptive, procedurally-integrated solutions, announced that it has received expanded 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the company's PRECICE system from NuVasive Specialized Orthopedics (NSO) with expanded indications that now include open and closed fracture fixation, pseudoarthrosis, malunions, nonunions, and bone transport.
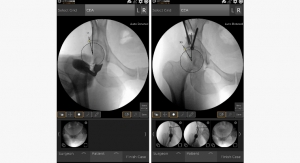
The PRECICE system is an innovative solution to treat patients with limb length discrepancy, limb deformities, and chronic nonunions. The platform consists of an intramedullary device that once implanted utilizes an external remote controller to non-invasively compress and distract long bones. The key to the PRECICE platform is the magnetic interaction between the PRECICE intramedullary nail and external remote control. The proprietary technology includes a complex internal gear system remotely activated and controlled by permanent magnets. This advancement in limb reconstruction allows for a precision controlled distraction phase with the ability to non-invasively customize treatment.
Prior to the expanded FDA clearance, the PRECICE system was indicated for limb lengthening of the femur and tibia. The system is now indicated for bone transport of long bones in addition to limb lengthening. Bone transport is a technique that allows for regeneration of bony tissue and is typically used to fill segmental bone loss due to trauma or infection, i.e. infected nonunions, segmental defect, and chronic bone infections. The expanded indications for use allows the company to continue to build a platform for further growth in the limb reconstruction and trauma markets.
"This FDA 510(k) clearance of PRECICE for expanded indications, including bone transport, demonstrates the evolving innovative capabilities of our technology to transform and expand the limb reconstruction and trauma markets," said Massimo Calafiore, president of NSO. "NSO remains committed to providing trauma surgeons with proper solutions to treat unmet clinical needs and challenging fractures. This allows us to treat more patients suffering from debilitating segmental bone defects through the use of PRECICE in bone transport procedures."
The company has also created a patient and family online resource dedicated to providing information on limb length discrepancy with Reach Your Height. The digital resource center provides education on treatment options and shares patient success stories. To learn more, visit reachyourheight.com.
The PRECICE system is an innovative solution to treat patients with limb length discrepancy, limb deformities, and chronic nonunions. The platform consists of an intramedullary device that once implanted utilizes an external remote controller to non-invasively compress and distract long bones. The key to the PRECICE platform is the magnetic interaction between the PRECICE intramedullary nail and external remote control. The proprietary technology includes a complex internal gear system remotely activated and controlled by permanent magnets. This advancement in limb reconstruction allows for a precision controlled distraction phase with the ability to non-invasively customize treatment.
Prior to the expanded FDA clearance, the PRECICE system was indicated for limb lengthening of the femur and tibia. The system is now indicated for bone transport of long bones in addition to limb lengthening. Bone transport is a technique that allows for regeneration of bony tissue and is typically used to fill segmental bone loss due to trauma or infection, i.e. infected nonunions, segmental defect, and chronic bone infections. The expanded indications for use allows the company to continue to build a platform for further growth in the limb reconstruction and trauma markets.
"This FDA 510(k) clearance of PRECICE for expanded indications, including bone transport, demonstrates the evolving innovative capabilities of our technology to transform and expand the limb reconstruction and trauma markets," said Massimo Calafiore, president of NSO. "NSO remains committed to providing trauma surgeons with proper solutions to treat unmet clinical needs and challenging fractures. This allows us to treat more patients suffering from debilitating segmental bone defects through the use of PRECICE in bone transport procedures."
The company has also created a patient and family online resource dedicated to providing information on limb length discrepancy with Reach Your Height. The digital resource center provides education on treatment options and shares patient success stories. To learn more, visit reachyourheight.com.