GE Additive03.09.18
Q: What is the new service and why are you launching it at AAOS 2018?
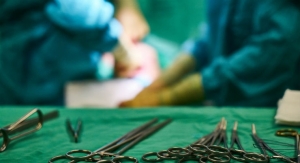
Ingvarsson: The medical technology sector is up there with the aerospace industry in terms of early adoption of additive manufacturing and pushing the boundaries of this emerging technology.
There are parallels in the way these two sectors are beginning to incorporate and scale additive manufacturing from a prototyping solution into more mainstream production—within a heavily regulated market.
We often hear from customers, particularly small to medium-sized medical device companies, that they need guidance and additional resources to help with equipment validation, testing and component verification and also to interpret regulatory and compliance requirements.
So, with that in mind we’ve developed the Orthopaedic Validation Consultancy (OVC) in conjunction with our colleagues at GE Additive AddWorks to guide our customers through the process.
As we’re always keen to get constructive input from orthopedic surgeons and the wider industry, we excited to announce this new service at AAOS 2018.
Q: Who is the OVC aimed at?
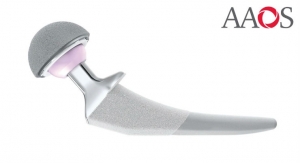
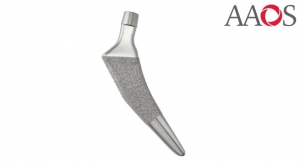
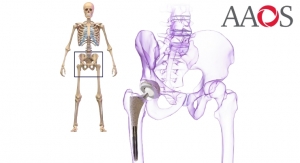
Ingvarsson: The service has been created for medical technology companies using our Q10plus machines that have to adhere to regulatory and compliancy requirements—be that FDA, ISO 13485 and other regional and global standards.
Our largest customers often have established teams and systems already in place to help steward them through the validation process, but we’re happy to see how this approach could enhance their process. However, we think the OVC with its step-by-step approach could add the most value to smaller device firms, start-ups or spin-offs that don’t always have resources or expertise in-house.
Q: How will the service be delivered?
Ingvarsson: It’s designed very much to be a collaborative process with our customers over a three to eight month period. Of course, timeframes will vary depending on each customer’s requirement and overall readiness.
But the desired outcome is twofold; firstly, increase time to market and help customers get into production with a validated machine and secondly, create value in a cost-efficient way. For some that might mean a faster production start, for others it might be guidance on powder selections and test builds, or even advice on how to develop a rigorous risk management strategy.
We have created a five-step approach from assessment through to validation. This way we can provide bespoke modularity and flexibility to map to each customer’s point on their additive journey.
With each customer, we create an understanding of the production process, identifying needs and critical parameters and what need to be tested and proven. Finding joint conclusions are the foundations of the process validation.
Q: Have any of your existing medical technology customers signed up?
Ingvarsson: Yes, in fact, we’re already working with a number of our customers—both large and small. Their response has been overwhelmingly positive.
Working with the Arcam team during the validation process, we hope that they will be able to acquire deep knowledge and in turn foster a strong additive manufacturing mind-set within their organizations.
Ingvarsson: The medical technology sector is up there with the aerospace industry in terms of early adoption of additive manufacturing and pushing the boundaries of this emerging technology.
There are parallels in the way these two sectors are beginning to incorporate and scale additive manufacturing from a prototyping solution into more mainstream production—within a heavily regulated market.
We often hear from customers, particularly small to medium-sized medical device companies, that they need guidance and additional resources to help with equipment validation, testing and component verification and also to interpret regulatory and compliance requirements.
So, with that in mind we’ve developed the Orthopaedic Validation Consultancy (OVC) in conjunction with our colleagues at GE Additive AddWorks to guide our customers through the process.
As we’re always keen to get constructive input from orthopedic surgeons and the wider industry, we excited to announce this new service at AAOS 2018.
Q: Who is the OVC aimed at?
Ingvarsson: The service has been created for medical technology companies using our Q10plus machines that have to adhere to regulatory and compliancy requirements—be that FDA, ISO 13485 and other regional and global standards.
Our largest customers often have established teams and systems already in place to help steward them through the validation process, but we’re happy to see how this approach could enhance their process. However, we think the OVC with its step-by-step approach could add the most value to smaller device firms, start-ups or spin-offs that don’t always have resources or expertise in-house.
Q: How will the service be delivered?
Ingvarsson: It’s designed very much to be a collaborative process with our customers over a three to eight month period. Of course, timeframes will vary depending on each customer’s requirement and overall readiness.
But the desired outcome is twofold; firstly, increase time to market and help customers get into production with a validated machine and secondly, create value in a cost-efficient way. For some that might mean a faster production start, for others it might be guidance on powder selections and test builds, or even advice on how to develop a rigorous risk management strategy.
We have created a five-step approach from assessment through to validation. This way we can provide bespoke modularity and flexibility to map to each customer’s point on their additive journey.
With each customer, we create an understanding of the production process, identifying needs and critical parameters and what need to be tested and proven. Finding joint conclusions are the foundations of the process validation.
Q: Have any of your existing medical technology customers signed up?
Ingvarsson: Yes, in fact, we’re already working with a number of our customers—both large and small. Their response has been overwhelmingly positive.
Working with the Arcam team during the validation process, we hope that they will be able to acquire deep knowledge and in turn foster a strong additive manufacturing mind-set within their organizations.