Jennifer Chu, MIT News Office06.27.18
Prosthetic limb technology has advanced by leaps and bounds, giving amputees a range of bionic options, including artificial knees controlled by microchips, sensor-laden feet driven by artificial intelligence, and robotic hands that a user can manipulate with her mind. But such high-tech designs can cost tens of thousands of dollars, making them unattainable for many amputees, particularly in developing countries.
Now MIT engineers have developed a simple, low-cost, passive prosthetic foot that they can tailor to an individual. Given a user’s body weight and size, the researchers can tune the shape and stiffness of the prosthetic foot, such that the user’s walk is similar to an able-bodied gait. They estimate that the foot, if manufactured on a wide scale, could cost an order of magnitude less than existing products.
The custom-designed prostheses are based on a design framework developed by the researchers, which provides a quantitative way to predict a user’s biomechanical performance, or walking behavior, based on the mechanical design of the prosthetic foot.
“[Walking] is something so core to us as humans, and for this segment of the population who have a lower-limb amputation, there’s just no theory for us to say, ‘here’s exactly how we should design the stiffness and geometry of a foot for you, in order for you to walk as you desire,’” said Amos Winter, associate professor of mechanical engineering at MIT. “Now we can do that. And that’s super powerful.”
Winter and former graduate student Kathryn Olesnavage reported details of this framework in IEEE’s Transactions on Neural Systems and Rehabilitation. They have published their results on their new prosthetic foot in the ASME Journal of Mechanical Design, with graduate student Victor Prost and research engineer William Brett Johnson.
In 2012, soon after Winter joined the MIT faculty, he was approached by Jaipur Foot, a manufacturer of artificial limbs based in Jaipur, India. The organization manufactures a passive prosthetic foot, geared toward amputees in developing countries, and donates more than 28,000 models each year to users in India and elsewhere.
“They’ve been making this foot for over 40 years, and it’s rugged, so farmers can use it barefoot outdoors, and it’s relatively life-like, so if people go in a mosque and want to pray barefoot, they’re likely to not be stigmatized,” Winter said. “But it’s quite heavy, and the internal structure is made all by hand, which creates a big variation in product quality.”
The organization asked Winter whether he could design a better, lighter foot that could be mass-produced at low cost.
“At that point, we started asking ourselves, ‘how should we design this foot as engineers? How should we predict the performance, given the foot’s stiffness and mechanical design and geometry? How should we tune all that to get a person to walk the way we want them to walk?’” Winter recalls.
The team, led by Olesnavage, first looked for a way to quantitatively relate a prosthesis’ mechanical characteristics to a user’s walking performance—a fundamental relationship that had never before been fully codified.
While many developers of prosthetic feet have focused on replicating the movements of able-bodied feet and ankles, Winter’s team took a different approach, based on their realization that amputees who have lost a limb below the knee can’t feel what a prosthetic foot does.
“One of the critical insights we had was that, to a user, the foot is just kind of like a black box—it’s not connected to their nervous system, and they’re not interacting with the foot intimately,” Winter said.
Instead of designing a prosthetic foot to replicate the motions of an able-bodied foot, he and Olesnavage looked to design a prosthetic foot that would produce lower-leg motions similar to those of an able-bodied person’s lower leg as they walk.
“This really opened up the design space for us,” Winter said. “We can potentially drastically change the foot, so long as we make the the lower leg do what we want it to do, in terms of kinematics and loading, because that’s what a user perceives.”
With the lower leg in mind, the team looked for ways to relate how the mechanics of the foot relate to how the lower leg moves while the foot is in contact with the ground. To do this, the researchers consulted an existing dataset comprising measurements of steps taken by an able-bodied walker with a given body size and weight. With each step, previous researchers had recorded the ground reaction forces and the changing center of pressure experienced by a walker’s foot as it rocked from heel to toe, along with the position and trajectory of the lower leg.
Winter and his colleagues developed a mathematical model of a simple, passive prosthetic foot, which describes the stiffness, possible motion, and shape of the foot. They plugged into the model the ground reaction forces from the dataset, which they could sum up to predict how a user’s lower leg would translate through a single step.
With their model, they then tuned the stiffness and geometry of the simulated prosthetic foot to produce a lower-leg trajectory that was close to the able-bodied swing—a measure they consider to be a minimal “lower leg trajectory error.”
“Ideally, we would tune the stiffness and geometry of the foot perfectly so we exactly replicate the motion of the lower leg,” Winter said. “Overall, we saw that we can get pretty darn close to able-bodied motion and loading, with a passive structure.”
Evolving on a Curve
The team then sought to identify an ideal shape for a single-part prosthetic foot that would be simple and affordable to manufacture, while still producing a leg trajectory very similar to that of able-bodied walkers.
To pinpoint an ideal foot shape, the group ran a “genetic algorithm”—a common technique used to weed out unfavorable options, in search of the most optimal designs.
“Just like a population of animals, we made a population of feet, all with different variables to make different curve shapes,” Winter said. “We loaded them into simulation and calculated their lower leg trajectory error. The ones that had a high error, we killed off.”
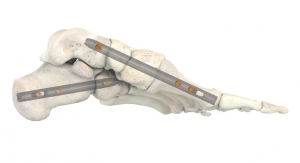
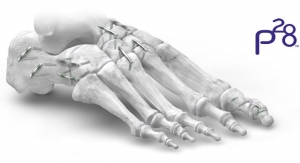
Those that had a lower error, the researchers further mixed and matched with other shapes, to evolve the population toward an ideal shape, with the lowest possible lower leg trajectory error. The team used a wide Bezier curve to describe the shape of the foot using only a few select variables, which were easy to vary in the genetic algorithm. The resulting foot shape looked similar to the side-view of a toboggan.
Olesnavage and Winter figured that, by tuning the stiffness and shape of this Bezier curve to a person’s body weight and size, the team should be able to produce a prosthetic foot that generates leg motions similar to able-bodied walking. To test this idea, the researchers produced several feet for volunteers in India. The prostheses were made from machined nylon, a material chosen for its energy-storage capability.
“What’s cool is, this behaves nothing like an able-bodied foot—there’s no ankle or metatarsal joint—it’s just one big structure, and all we care about is how the lower leg is moving through space,” Winter said. “Most of the testing was done indoors, but one guy ran outside, he liked it so much. It puts a spring in your step.”
Going forward, the team has partnered with Vibram, an Italian company that manufactures rubber outsoles—flexible hiking boots and running shoes that look like feet. The company is designing a life-like covering for the team’s prosthesis, that will also give the foot some traction over muddy or slippery surfaces. The researchers plan to test the prosthetics and coverings on volunteers in India this spring.
Winter said the simple prosthetic foot design can also be a much more affordable and durable option for populations such as soldiers who want to return to active duty or veterans who want to live an active lifestyle.
“A common passive foot in the U.S. market will cost $1,000 to $10,000, made out of carbon fiber. Imagine you go to your prosthetist, they take a few measurements, they send them back to us, and we send back to you a custom-designed nylon foot for a few hundred bucks. This model is potentially game-changing for the industry, because we can fully quantify the foot and tune it for individuals, and use cheaper materials.”
This research was funded, in part, by the MIT Tata Center for Technology and Design.
Now MIT engineers have developed a simple, low-cost, passive prosthetic foot that they can tailor to an individual. Given a user’s body weight and size, the researchers can tune the shape and stiffness of the prosthetic foot, such that the user’s walk is similar to an able-bodied gait. They estimate that the foot, if manufactured on a wide scale, could cost an order of magnitude less than existing products.
The custom-designed prostheses are based on a design framework developed by the researchers, which provides a quantitative way to predict a user’s biomechanical performance, or walking behavior, based on the mechanical design of the prosthetic foot.
“[Walking] is something so core to us as humans, and for this segment of the population who have a lower-limb amputation, there’s just no theory for us to say, ‘here’s exactly how we should design the stiffness and geometry of a foot for you, in order for you to walk as you desire,’” said Amos Winter, associate professor of mechanical engineering at MIT. “Now we can do that. And that’s super powerful.”
Winter and former graduate student Kathryn Olesnavage reported details of this framework in IEEE’s Transactions on Neural Systems and Rehabilitation. They have published their results on their new prosthetic foot in the ASME Journal of Mechanical Design, with graduate student Victor Prost and research engineer William Brett Johnson.
In 2012, soon after Winter joined the MIT faculty, he was approached by Jaipur Foot, a manufacturer of artificial limbs based in Jaipur, India. The organization manufactures a passive prosthetic foot, geared toward amputees in developing countries, and donates more than 28,000 models each year to users in India and elsewhere.
“They’ve been making this foot for over 40 years, and it’s rugged, so farmers can use it barefoot outdoors, and it’s relatively life-like, so if people go in a mosque and want to pray barefoot, they’re likely to not be stigmatized,” Winter said. “But it’s quite heavy, and the internal structure is made all by hand, which creates a big variation in product quality.”
The organization asked Winter whether he could design a better, lighter foot that could be mass-produced at low cost.
“At that point, we started asking ourselves, ‘how should we design this foot as engineers? How should we predict the performance, given the foot’s stiffness and mechanical design and geometry? How should we tune all that to get a person to walk the way we want them to walk?’” Winter recalls.
The team, led by Olesnavage, first looked for a way to quantitatively relate a prosthesis’ mechanical characteristics to a user’s walking performance—a fundamental relationship that had never before been fully codified.
While many developers of prosthetic feet have focused on replicating the movements of able-bodied feet and ankles, Winter’s team took a different approach, based on their realization that amputees who have lost a limb below the knee can’t feel what a prosthetic foot does.
“One of the critical insights we had was that, to a user, the foot is just kind of like a black box—it’s not connected to their nervous system, and they’re not interacting with the foot intimately,” Winter said.
Instead of designing a prosthetic foot to replicate the motions of an able-bodied foot, he and Olesnavage looked to design a prosthetic foot that would produce lower-leg motions similar to those of an able-bodied person’s lower leg as they walk.
“This really opened up the design space for us,” Winter said. “We can potentially drastically change the foot, so long as we make the the lower leg do what we want it to do, in terms of kinematics and loading, because that’s what a user perceives.”
With the lower leg in mind, the team looked for ways to relate how the mechanics of the foot relate to how the lower leg moves while the foot is in contact with the ground. To do this, the researchers consulted an existing dataset comprising measurements of steps taken by an able-bodied walker with a given body size and weight. With each step, previous researchers had recorded the ground reaction forces and the changing center of pressure experienced by a walker’s foot as it rocked from heel to toe, along with the position and trajectory of the lower leg.
Winter and his colleagues developed a mathematical model of a simple, passive prosthetic foot, which describes the stiffness, possible motion, and shape of the foot. They plugged into the model the ground reaction forces from the dataset, which they could sum up to predict how a user’s lower leg would translate through a single step.
With their model, they then tuned the stiffness and geometry of the simulated prosthetic foot to produce a lower-leg trajectory that was close to the able-bodied swing—a measure they consider to be a minimal “lower leg trajectory error.”
“Ideally, we would tune the stiffness and geometry of the foot perfectly so we exactly replicate the motion of the lower leg,” Winter said. “Overall, we saw that we can get pretty darn close to able-bodied motion and loading, with a passive structure.”
Evolving on a Curve
The team then sought to identify an ideal shape for a single-part prosthetic foot that would be simple and affordable to manufacture, while still producing a leg trajectory very similar to that of able-bodied walkers.
To pinpoint an ideal foot shape, the group ran a “genetic algorithm”—a common technique used to weed out unfavorable options, in search of the most optimal designs.
“Just like a population of animals, we made a population of feet, all with different variables to make different curve shapes,” Winter said. “We loaded them into simulation and calculated their lower leg trajectory error. The ones that had a high error, we killed off.”
Those that had a lower error, the researchers further mixed and matched with other shapes, to evolve the population toward an ideal shape, with the lowest possible lower leg trajectory error. The team used a wide Bezier curve to describe the shape of the foot using only a few select variables, which were easy to vary in the genetic algorithm. The resulting foot shape looked similar to the side-view of a toboggan.
Olesnavage and Winter figured that, by tuning the stiffness and shape of this Bezier curve to a person’s body weight and size, the team should be able to produce a prosthetic foot that generates leg motions similar to able-bodied walking. To test this idea, the researchers produced several feet for volunteers in India. The prostheses were made from machined nylon, a material chosen for its energy-storage capability.
“What’s cool is, this behaves nothing like an able-bodied foot—there’s no ankle or metatarsal joint—it’s just one big structure, and all we care about is how the lower leg is moving through space,” Winter said. “Most of the testing was done indoors, but one guy ran outside, he liked it so much. It puts a spring in your step.”
Going forward, the team has partnered with Vibram, an Italian company that manufactures rubber outsoles—flexible hiking boots and running shoes that look like feet. The company is designing a life-like covering for the team’s prosthesis, that will also give the foot some traction over muddy or slippery surfaces. The researchers plan to test the prosthetics and coverings on volunteers in India this spring.
Winter said the simple prosthetic foot design can also be a much more affordable and durable option for populations such as soldiers who want to return to active duty or veterans who want to live an active lifestyle.
“A common passive foot in the U.S. market will cost $1,000 to $10,000, made out of carbon fiber. Imagine you go to your prosthetist, they take a few measurements, they send them back to us, and we send back to you a custom-designed nylon foot for a few hundred bucks. This model is potentially game-changing for the industry, because we can fully quantify the foot and tune it for individuals, and use cheaper materials.”
This research was funded, in part, by the MIT Tata Center for Technology and Design.