GlobeNewswire11.20.18
Aurora Spine Corporation announced that it has acquired an exclusive license to US patent #9,451,986 titled “Percutaneous sacroiliac joint implant and method for surgically inserting and securing the implant into the sacroiliac joint” in an agreement with SILIF Corporation of Buffalo, N.Y., the inventor of a posterior SI Fusion technology.
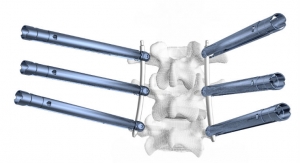
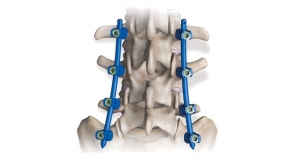
The license will enable Aurora Spine to develop a new Posterior Sacroiliac Joint Fixation System. The implant, named EASiFX, will feature multiple retaining and bone locking mechanisms. The EASiFX procedure will offer less tissue disruption, decreased operating room time and radiation exposure. The procedure also offers minimal distraction of the SI joint through a safe, posterior method.
The license is another example of Aurora Spine’s vision and leadership in the provision of fusion solutions for a diverse range of spine surgery procedures. EASiFX is expected to add to the company’s expanding product portfolio of unique implant products and add to the growth of Aurora Spine.
“Aurora Spine is changing spine surgery. The SILIF patent technology, which has been enthusiastically received by surgeons, has the potential to become the standard of care for spine patients with SI Joint pathologies,” said Trent Northcutt, president and CEO of Aurora Spine. “The license provides access to a procedure that has been growing in excess of 20 percent in recent years, leverages the core competencies and vision of our minimally invasive spine portfolio of game-changing innovative products and provides new growth opportunities in the U.S. We believe this opportunity is perfectly aligned with our spine-focused expansion strategy to pursue niche differentiated products, customer retention, and support portfolio pull-through along the way.”
“The EASiFX implant is a differentiated product designed to drive fusion and is supported by an expanding market segment. We remain committed to growing our spine portfolio and infusing our expertise to help our hospital, surgeon and distribution partners,” said Northcutt.
“The EASiFX implant prototypes were successfully used in a cadaver lab setting earlier this year along with only a few surgical tools required to complete a successful implant placement,” said Laszlo Garamszegi, chief technology officer of Aurora Spine. “The implants performed remarkably well in our test and we are looking forward to presenting all the benefits the invention offers to patients and physicians.”
According to iData Research, the U.S. minimally invasive spinal implants market is expected to increase to over $2 billion by 2023. The fastest growing implant segment is the MIS sacroiliac joint fusion market. Clinical evidence demonstrates that sacroiliac joint fusion (“SIJF”) is an effective procedure. The popularity of SIJF has grown rapidly over the past five years. The SIJF market is currently 5 percent of the overall spine market and it’s projected to reach 20 percent by 2021. By 2024, the SIJF market in the US is expected to exceed $200 million, driven primarily by an increase in procedure volumes and adoption by new surgeons.
As consideration for the license, the company will issue 1,000,000 common shares of the company to SILIF at a price of CDN$0.30 per share, with all such shares being subject to a 5 year tiered lock-up agreement, with 20 percent of the shares released from the lock-up on each anniversary of the closing date of the transaction. In addition, the company will issue SILIF warrants to purchase up to 1,750,000 common shares of the company, exercisable at CDN$0.35 for a period of 5 years following the date of grant. The warrants will vest in 20 percent increments on each anniversary of the closing date of the transaction.
All securities issued by Aurora Spine in connection with the transaction will be subject to a statutory four-month hold period. The transaction is subject to certain conditions including, but not limited to, the receipt of all necessary approvals, including the approval of the TSX Venture Exchange.
The license will enable Aurora Spine to develop a new Posterior Sacroiliac Joint Fixation System. The implant, named EASiFX, will feature multiple retaining and bone locking mechanisms. The EASiFX procedure will offer less tissue disruption, decreased operating room time and radiation exposure. The procedure also offers minimal distraction of the SI joint through a safe, posterior method.
The license is another example of Aurora Spine’s vision and leadership in the provision of fusion solutions for a diverse range of spine surgery procedures. EASiFX is expected to add to the company’s expanding product portfolio of unique implant products and add to the growth of Aurora Spine.
“Aurora Spine is changing spine surgery. The SILIF patent technology, which has been enthusiastically received by surgeons, has the potential to become the standard of care for spine patients with SI Joint pathologies,” said Trent Northcutt, president and CEO of Aurora Spine. “The license provides access to a procedure that has been growing in excess of 20 percent in recent years, leverages the core competencies and vision of our minimally invasive spine portfolio of game-changing innovative products and provides new growth opportunities in the U.S. We believe this opportunity is perfectly aligned with our spine-focused expansion strategy to pursue niche differentiated products, customer retention, and support portfolio pull-through along the way.”
“The EASiFX implant is a differentiated product designed to drive fusion and is supported by an expanding market segment. We remain committed to growing our spine portfolio and infusing our expertise to help our hospital, surgeon and distribution partners,” said Northcutt.
“The EASiFX implant prototypes were successfully used in a cadaver lab setting earlier this year along with only a few surgical tools required to complete a successful implant placement,” said Laszlo Garamszegi, chief technology officer of Aurora Spine. “The implants performed remarkably well in our test and we are looking forward to presenting all the benefits the invention offers to patients and physicians.”
According to iData Research, the U.S. minimally invasive spinal implants market is expected to increase to over $2 billion by 2023. The fastest growing implant segment is the MIS sacroiliac joint fusion market. Clinical evidence demonstrates that sacroiliac joint fusion (“SIJF”) is an effective procedure. The popularity of SIJF has grown rapidly over the past five years. The SIJF market is currently 5 percent of the overall spine market and it’s projected to reach 20 percent by 2021. By 2024, the SIJF market in the US is expected to exceed $200 million, driven primarily by an increase in procedure volumes and adoption by new surgeons.
As consideration for the license, the company will issue 1,000,000 common shares of the company to SILIF at a price of CDN$0.30 per share, with all such shares being subject to a 5 year tiered lock-up agreement, with 20 percent of the shares released from the lock-up on each anniversary of the closing date of the transaction. In addition, the company will issue SILIF warrants to purchase up to 1,750,000 common shares of the company, exercisable at CDN$0.35 for a period of 5 years following the date of grant. The warrants will vest in 20 percent increments on each anniversary of the closing date of the transaction.
All securities issued by Aurora Spine in connection with the transaction will be subject to a statutory four-month hold period. The transaction is subject to certain conditions including, but not limited to, the receipt of all necessary approvals, including the approval of the TSX Venture Exchange.