Sam Brusco, Associate Editor09.02.22
French company eCential Robotics has earned U.S. Food and Drug Administration (FDA) 510(k) clearance for its 3D imaging, navigation, and robotics guidance system for spine surgery.
eCential aims to simplify robotic-assisted bone surgery by focusing workflows on the essential. The system is fully open, meaning it can be used with any manufacturer’s implants. The company reportedly welcomed implant makers to create specific “Apps” for its system for multiple indications, most notably spine, cranial, traumatology, orthopedics, and sports medicine.
The system is built around a range of Apps currently dedicated to spine surgery, but the company aims to extend the platform to multiple bone surgery applications.
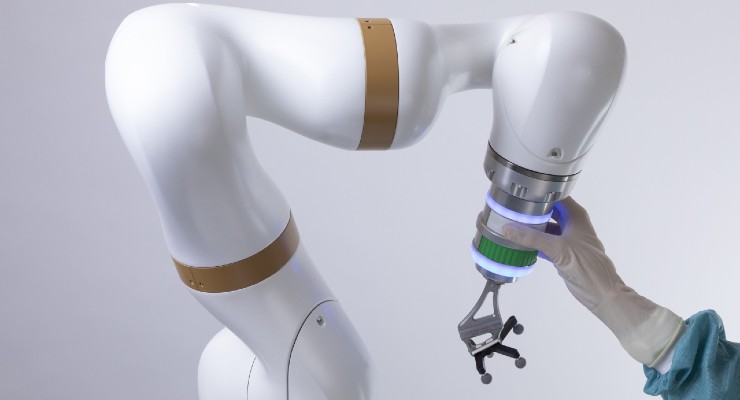
The robotic arm of the surgical robotics platform that received FDA certification. Image courtesy of eCential Robotics.
Since the company’s first platform was commercialized in Europe, ten units were installed and used in over 2,000 surgeries. eCential’s U.S. subsidiary was established in December 2020. In Q1 2022, the firm conducted preclinical evaluation tests in the U.S.
According to the company, this landmark FDA clearance represents the first universal application for an intra-operative 2D and 3D imaging, navigation, and robotic guidance system for spine surgery. The system avoids the typical pitfalls of image-navigation pairing like unreliable calibration and registration steps. The system also automates many technical steps, with a single interface to control imaging, navigation, and robotic functions.
The unified open platform features 2D/3D medical imaging and stereotaxic guidance. It consists of a mobile C-arm, mobile viewing workstation, and mobile collaborative robot (cobot). The mobile X-ray is an imaging robot with 5-axis that supports the 2D/3D imaging. 3D optical position tracking enables both freehand navigation and robotic guidance via a stereoscopic camera, computer platform with monitors, navigation software, a robotic arm, and instruments with marker spheres to pinpoint localization.
"The FDA clearance of the eCential Robotics unified platform recognizes reliability and robustness of our product, confirms the confidence in eCential Robotics' unique concept of focusing surgical workflows on the essential via a unified, open and multi-app system, and also encourages our ambition to expand our footprint in the United States," Laurence Chabanas, eCential Robotics’ chief strategy officer and USA CEO, told the press. "This 510(k) is fundamental to our strategy. We are excited about these bold new and disruptive technologies and the role that eCential Robotics can play in reshaping bone surgical procedures and restoring patients' quality of life," she added.
eCential Robotics will be exhibiting at this year's North American Spine Society (NASS) annual meeting. The company began partnering with ChoiceSpine to use its implant portfolio with the robotic solution in June.
eCential aims to simplify robotic-assisted bone surgery by focusing workflows on the essential. The system is fully open, meaning it can be used with any manufacturer’s implants. The company reportedly welcomed implant makers to create specific “Apps” for its system for multiple indications, most notably spine, cranial, traumatology, orthopedics, and sports medicine.
The system is built around a range of Apps currently dedicated to spine surgery, but the company aims to extend the platform to multiple bone surgery applications.

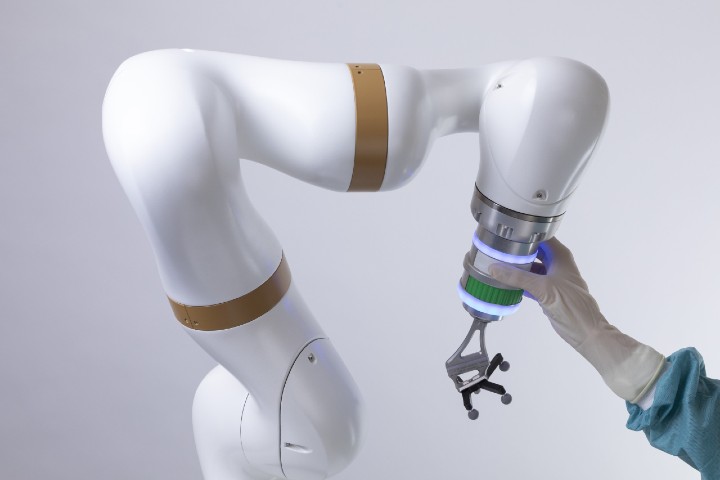
The robotic arm of the surgical robotics platform that received FDA certification. Image courtesy of eCential Robotics.
Since the company’s first platform was commercialized in Europe, ten units were installed and used in over 2,000 surgeries. eCential’s U.S. subsidiary was established in December 2020. In Q1 2022, the firm conducted preclinical evaluation tests in the U.S.
According to the company, this landmark FDA clearance represents the first universal application for an intra-operative 2D and 3D imaging, navigation, and robotic guidance system for spine surgery. The system avoids the typical pitfalls of image-navigation pairing like unreliable calibration and registration steps. The system also automates many technical steps, with a single interface to control imaging, navigation, and robotic functions.
The unified open platform features 2D/3D medical imaging and stereotaxic guidance. It consists of a mobile C-arm, mobile viewing workstation, and mobile collaborative robot (cobot). The mobile X-ray is an imaging robot with 5-axis that supports the 2D/3D imaging. 3D optical position tracking enables both freehand navigation and robotic guidance via a stereoscopic camera, computer platform with monitors, navigation software, a robotic arm, and instruments with marker spheres to pinpoint localization.
"The FDA clearance of the eCential Robotics unified platform recognizes reliability and robustness of our product, confirms the confidence in eCential Robotics' unique concept of focusing surgical workflows on the essential via a unified, open and multi-app system, and also encourages our ambition to expand our footprint in the United States," Laurence Chabanas, eCential Robotics’ chief strategy officer and USA CEO, told the press. "This 510(k) is fundamental to our strategy. We are excited about these bold new and disruptive technologies and the role that eCential Robotics can play in reshaping bone surgical procedures and restoring patients' quality of life," she added.
eCential Robotics will be exhibiting at this year's North American Spine Society (NASS) annual meeting. The company began partnering with ChoiceSpine to use its implant portfolio with the robotic solution in June.