Sam Brusco, Associate Editor02.12.18
If a roadway is cracked and full of potholes, it’s fixed by repaving. If an edifice is damaged, it’s fixed by rebuilding. But if a joint in the body is damaged, repair isn’t quite so simple.
The meniscus, for example—the cartilage cushion between the thighbone and shinbone—is commonly torn during all kinds of activities, from running to shoveling snow during New Jersey winters. (Pretty much anything involving activities that put pressure on or rotate the knee joint can potentially cause damage to the meniscus.) The dense extracellular matrix in deep tissues like the meniscus makes it difficult for injuries to heal themselves, and meniscus surgery is not really a “repair” job. The procedure—one of the most common orthopedic surgeries—involves removing the torn part of the meniscus, leaving behind less of it to bear the same load. (Imagine repairing structural damage to a building by simply cutting away the broken parts. The whole thing would come tumbling down).
For less invasive procedures to become viable to patch up the meniscus, the cells in and around the area must be encouraged to repair themselves. Penn scientists are using a tissue engineering strategy to improve healing related to the skeletal system, aiming to “blend biology with engineering to understand normal and pathologic processes to ultimately replace or regenerate musculoskeletal tissues,” as Lou Soslowsky, Ph.D., Penn’s vice chair for research in the department of Orthopaedic Surgery, explains.
The meniscus’ cellular matrix is replete with proteins and sugars filling the spaces between cells, binding tissues together. Due to this, it’s difficult for stem cells to migrate to the injury site and begin repairing.
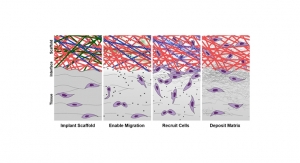
To face this challenge, Robert Mauck, Ph.D., a professor of Orthopaedic Surgery, and Jason Burdick, Ph.D., a professor of Bioengineering, are testing a microscopic scaffold with a growth factor to attract stem cells, laced with an enzyme to loosen the troublesome matrix. Upon placement in the meniscus tissue of a cow, the matrix complied and bone stem cells migrated to the scaffold to begin the reconstruction.
“The immediate next step is to test this system in a large animal model to see if it can repair a meniscus tear,” Mauck said. “This approach could possibly be in human trials in about five years.”
Articular cartilage is another problem spot for healing—it doesn’t have a blood supply and thus has a very limited capacity to regrow. There have yet to be any effective treatment methods, and even therapies using stem cells and tissue-engineered techniques have yielded poor regenerative repair quality and tissue integration-to-lesion base.
In response to this, Osaka University researchers invented synthetic tissue using synovium-derived mesenchymal stem cells (MSCs). The first in-human trial and surgery on the first patient was performed in early January as part of a Phase III clinical study taking place in Osaka University Hospital. It was the first regenerative therapy clinical trial of its kind in Japan, and combined allogeneic stem cells and commercial use of a Medical Center for Translational Research (MTR) of Osaka University stem cell bank.
Osaka University researchers Norimasa Nakamura, Hideki Yoshikawa, and Yoshiki Sawa developed the 3D synthetic material using only MSCs as the starting material. Featuring excellent differentiation ability and tissue adhesive properties, the synthetic tissue’s properties can be transplanted via minimally invasive approaches like arthroscopy. According to the three researchers, the scaffold-free tissue engineering technique demonstrates an advantage in safety and cost effectiveness as well as tissue plasticity and adhesive properties on the cartilage surface.
Further, animal lovers can rejoice that the regenerative cartilage repair method doesn’t use animal-derived materials. It’s also the first clinical trial for regenerative tissue repair recruiting mega pharmaceutical companies—both Twocells Company Ltd. and Chugai Pharmaceutical Co. Ltd. participated in the trial.
Only one operation is necessary to complete the procedure, as opposed to the previous autologous implantation method requiring two to accomplish the treatment. It could potentially allow a treatment for athletic injuries as well as the early phases of degenerative joint diseases. The researchers anticipate the therapy could prevent the onset of osteoarthritis in middle-aged generations.
Before readers wonder whether regenerative approaches such as these are still muddled in university-stage trials, regenerative medicine company Orthocell Ltd. was granted a European patent titled, “Culture medium, culturing method, and use of tenocytes” in mid-December 2017. (Tenocytes are tendon regeneration cells.)
The patent involves a manufacturing method for these cells to create the Autologous Tenocyte Implantation (Ortho-ATI) product. The new patent gives Orthocell global IP protection—Orthocell’s tendon repair applications are now granted in the European Union, United States, China, Australia, Singapore, Hong Kong, and New Zealand.
With manufacturing facilities in place, proven safety and efficacy, and published clinical data, Orthocell stands poised to commercialize Ortho-ATI. It is an autologous and homogolous cellular approach—tendon cells harvested for multiplication are from the patient’s own healthy tendon.
The meniscus, for example—the cartilage cushion between the thighbone and shinbone—is commonly torn during all kinds of activities, from running to shoveling snow during New Jersey winters. (Pretty much anything involving activities that put pressure on or rotate the knee joint can potentially cause damage to the meniscus.) The dense extracellular matrix in deep tissues like the meniscus makes it difficult for injuries to heal themselves, and meniscus surgery is not really a “repair” job. The procedure—one of the most common orthopedic surgeries—involves removing the torn part of the meniscus, leaving behind less of it to bear the same load. (Imagine repairing structural damage to a building by simply cutting away the broken parts. The whole thing would come tumbling down).
For less invasive procedures to become viable to patch up the meniscus, the cells in and around the area must be encouraged to repair themselves. Penn scientists are using a tissue engineering strategy to improve healing related to the skeletal system, aiming to “blend biology with engineering to understand normal and pathologic processes to ultimately replace or regenerate musculoskeletal tissues,” as Lou Soslowsky, Ph.D., Penn’s vice chair for research in the department of Orthopaedic Surgery, explains.
The meniscus’ cellular matrix is replete with proteins and sugars filling the spaces between cells, binding tissues together. Due to this, it’s difficult for stem cells to migrate to the injury site and begin repairing.
To face this challenge, Robert Mauck, Ph.D., a professor of Orthopaedic Surgery, and Jason Burdick, Ph.D., a professor of Bioengineering, are testing a microscopic scaffold with a growth factor to attract stem cells, laced with an enzyme to loosen the troublesome matrix. Upon placement in the meniscus tissue of a cow, the matrix complied and bone stem cells migrated to the scaffold to begin the reconstruction.
“The immediate next step is to test this system in a large animal model to see if it can repair a meniscus tear,” Mauck said. “This approach could possibly be in human trials in about five years.”
Articular cartilage is another problem spot for healing—it doesn’t have a blood supply and thus has a very limited capacity to regrow. There have yet to be any effective treatment methods, and even therapies using stem cells and tissue-engineered techniques have yielded poor regenerative repair quality and tissue integration-to-lesion base.
In response to this, Osaka University researchers invented synthetic tissue using synovium-derived mesenchymal stem cells (MSCs). The first in-human trial and surgery on the first patient was performed in early January as part of a Phase III clinical study taking place in Osaka University Hospital. It was the first regenerative therapy clinical trial of its kind in Japan, and combined allogeneic stem cells and commercial use of a Medical Center for Translational Research (MTR) of Osaka University stem cell bank.
Osaka University researchers Norimasa Nakamura, Hideki Yoshikawa, and Yoshiki Sawa developed the 3D synthetic material using only MSCs as the starting material. Featuring excellent differentiation ability and tissue adhesive properties, the synthetic tissue’s properties can be transplanted via minimally invasive approaches like arthroscopy. According to the three researchers, the scaffold-free tissue engineering technique demonstrates an advantage in safety and cost effectiveness as well as tissue plasticity and adhesive properties on the cartilage surface.
Further, animal lovers can rejoice that the regenerative cartilage repair method doesn’t use animal-derived materials. It’s also the first clinical trial for regenerative tissue repair recruiting mega pharmaceutical companies—both Twocells Company Ltd. and Chugai Pharmaceutical Co. Ltd. participated in the trial.
Only one operation is necessary to complete the procedure, as opposed to the previous autologous implantation method requiring two to accomplish the treatment. It could potentially allow a treatment for athletic injuries as well as the early phases of degenerative joint diseases. The researchers anticipate the therapy could prevent the onset of osteoarthritis in middle-aged generations.
Before readers wonder whether regenerative approaches such as these are still muddled in university-stage trials, regenerative medicine company Orthocell Ltd. was granted a European patent titled, “Culture medium, culturing method, and use of tenocytes” in mid-December 2017. (Tenocytes are tendon regeneration cells.)
The patent involves a manufacturing method for these cells to create the Autologous Tenocyte Implantation (Ortho-ATI) product. The new patent gives Orthocell global IP protection—Orthocell’s tendon repair applications are now granted in the European Union, United States, China, Australia, Singapore, Hong Kong, and New Zealand.
With manufacturing facilities in place, proven safety and efficacy, and published clinical data, Orthocell stands poised to commercialize Ortho-ATI. It is an autologous and homogolous cellular approach—tendon cells harvested for multiplication are from the patient’s own healthy tendon.