Sean Fenske, Editor03.08.17
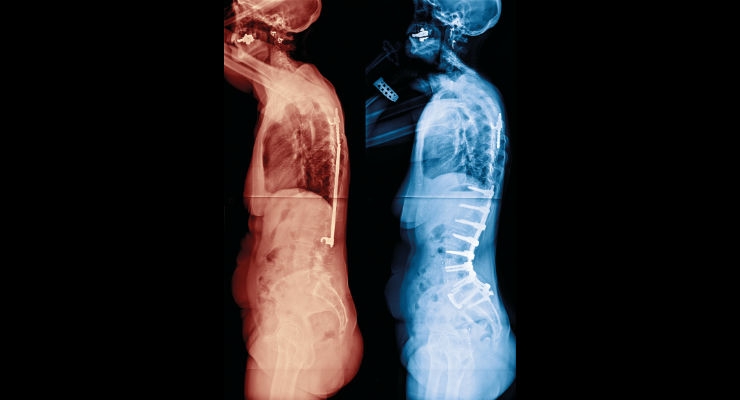
Personalized medicine has a different meaning for different members of the healthcare industry. In the orthopedics sector, however, it could mean custom implants and guides for a patient requiring a knee replacement. New manufacturing technologies coupled with patient scans enable device manufacturers to provide a solution that’s intended for a single specific patient.
That specialized capability has now been brought to the spinal fusion arena. Medicrea offers its patient-specific UNiD Rod technology, which represents a complete solution for spine surgeons. Richard Kienzle, the chief commercial and business development officer at the company took time to speak with ODT about the technology and the impact it is having on the industry.
Sean Fenske: Can you please take a moment to explain your patient-specific UNiD Rod technology? These are fabricated using actual patient imaging?
Richard Kienzle: UNiD Rods are generated as part of our specialized seven-step UNiD program that Medicrea has developed to drive our unique patient-centric approach to personalized spinal surgery. It allows us to work directly with surgeons to provide a custom treatment modality for each patient. The patient-specific UNiD Rod is defined in the preoperative predictive planning phase of this process and produced using a proprietary industrial contouring process. Our UNiD Lab team of specially trained biomechanical engineers measures the patient’s unique spinopelvic parameters from images of their spine to design a customized implant using clinically-evidenced sagittal alignment algorithms.
Fenske: Last year, you announced that your patient specific UNiD Rods for spinal fusion now come with a lifetime warranty. Can you tell me how you’re able to offer this first-of-its-kind guarantee?
Kienzle: We have now gone through more than 1,100 iterations of the UNiD program to generate a custom UNiD Rod for each patient. Our results from those procedures and all the data analyses that we've completed since are really strong. The UNiD Rod is performing at a rate that has enabled us to make this announcement and put this lifetime warranty in place. When you take a personalized approach to spinal surgery and follow the UNiD program from start to finish, you are creating an iterative feedback loop. UNiD removes a large degree of variability that is present in traditional spine surgery, and we are proud to stand by the program through a warranty on the UNiD Rod.
Fenske: More than 1,100 custom rods? That’s fantastic. What’s been the response from the surgeons working with this technology?

Richard Kienzle is the chief commercial and business development officer at Medicrea.
Kienzle: Surgeons have been very receptive to UNiD technology. We share a common goal of using the latest technologies and scientific research to improve patient outcomes. Medicrea galvanizes surgeons with an iterative process that has both predictive and deep learning capacity, delivering data and information that support surgical case decisions from pre-operative planning to postoperative analysis.
Fenske: In addition to providing the actual rods, how else are you collaborating with surgeons to enhance your solution?
Kienzle: The implant is just one piece of the puzzle here; UNiD represents a comprehensive service run through our UNiD Lab engineers who provide one-on-one dedicated support and value-add services to the entire process. Every surgery is an opportunity to improve surgeon experience and patient outcome. More and more spine surgeons are taking notice of Medicrea’s unique personalized approach. The UNiD program offers preoperative planning services; custom rod delivery based on the patient's plan; and a postoperative confirmation that the surgeon achieved the plan and restored the patient's unique sagittal balance.
Fenske: Since the rods are patient specific, what benefits are achieved during the procedure with regard to operating room time?
Kienzle: Surgeons were already “customizing” their rods but it happened during the operation using very basic bending tools, resulting in approximation in shape, loss of critical operating time, and even material damage—a known cause of postoperative implant failure. Essentially, surgeons were left to perform the last step in the implant manufacturing process without any support from device manufacturers to achieve their operative goals. With this hole in support, patient outcomes are clearly compromised. Medicrea stepped up to the plate to develop a patient-specific, outcome-focused solution with UNiD.
Fenske: It sounds like there is a combination of factors that all contribute to the success of the system that enables you to make the warranty offer. Would it be accurate to say there’s a natural synergy being created with each case between the company, the surgeon, the rods, the patient, the scans, the instruments, and the pre- and postoperative procedures?
Kienzle: There is certainly a synergy here that enables the UNiD program to simultaneously drive a value-based approach using personalized, data-driven medicine. What we have learned so far in pioneering this space in spine has convinced us that strategic surgical planning and analysis are key elements of a successful spinal surgery. Each time we collaborate with a surgeon, we are also benefiting by building a larger and larger data set that can be used to define the optimum strategy for the next individual patient. The data-driven UNiD program possesses an iterative learning capacity, so the more the surgeon uses it, the more data will be available to benchmark the next procedures. In essence, the system has a self-improvement mechanism, learning with each use.
Fenske: What do you foresee with regard to patient specific technology in the orthopedic space in the future? What’s coming five to ten years from now?
Kienzle: This is a busy and exciting time for us. We are continuing to expand the range of custom implants that can be generated through the UNiD program, while tracking a rapid uptake of the UNiD Rods in new and existing markets. We have already used the UNiD program to create custom 3D-printed interbody implants in Europe and expect to receive FDA clearance for the U.S. market before the end of 2017. We are also looking closely at using the program to develop new inventory control modalities that will significantly reduce the number of implants required to cover a typical spine surgery. The UNiD Lab will continue to be at the center of this expansion offering their comprehensive services and leading expertise to surgeons worldwide. As the custodians of personalized spine, we are hard at work ushering in a new era in patient care.
Fenske: Any other comments or thoughts you’d like to share?
Kienzle: Medicrea is pioneering a unique path that positions the company among the leading healthcare information technology firms and the next generation of outcome-driven device manufacturers. We are laser-focused on improving patient outcomes and providing system-wide cost reductions through a program of extensive clinical data compilation and mining, deep learning algorithms, and predictive analytics that culminates in precise and adaptive personalized spine care. In capturing a systems-based model for the iterative application of patient-specific spinal technology via UNiD, we are working to empirically answer the most difficult and complex clinical questions while providing strong, tangible value for healthcare shareholders benefiting patients, surgeons, hospitals, and payors in the process.
Because of this disruptive approach, we are neither restricted by nor economically dependent upon antiquated product revenue streams built upon clinical assumptions. We are also free of legacy manufacturing methods with their famously bloated distribution infrastructures. The 20th century medical device commercial concept that one size fits all and more is better does not live in our approach to spine. UNiD allows us to tackle tough, clinical questions and deploy sophisticated technology to validate patient outcomes, regardless of what the product technology or service solution need be.
That specialized capability has now been brought to the spinal fusion arena. Medicrea offers its patient-specific UNiD Rod technology, which represents a complete solution for spine surgeons. Richard Kienzle, the chief commercial and business development officer at the company took time to speak with ODT about the technology and the impact it is having on the industry.
Sean Fenske: Can you please take a moment to explain your patient-specific UNiD Rod technology? These are fabricated using actual patient imaging?
Richard Kienzle: UNiD Rods are generated as part of our specialized seven-step UNiD program that Medicrea has developed to drive our unique patient-centric approach to personalized spinal surgery. It allows us to work directly with surgeons to provide a custom treatment modality for each patient. The patient-specific UNiD Rod is defined in the preoperative predictive planning phase of this process and produced using a proprietary industrial contouring process. Our UNiD Lab team of specially trained biomechanical engineers measures the patient’s unique spinopelvic parameters from images of their spine to design a customized implant using clinically-evidenced sagittal alignment algorithms.
Fenske: Last year, you announced that your patient specific UNiD Rods for spinal fusion now come with a lifetime warranty. Can you tell me how you’re able to offer this first-of-its-kind guarantee?
Kienzle: We have now gone through more than 1,100 iterations of the UNiD program to generate a custom UNiD Rod for each patient. Our results from those procedures and all the data analyses that we've completed since are really strong. The UNiD Rod is performing at a rate that has enabled us to make this announcement and put this lifetime warranty in place. When you take a personalized approach to spinal surgery and follow the UNiD program from start to finish, you are creating an iterative feedback loop. UNiD removes a large degree of variability that is present in traditional spine surgery, and we are proud to stand by the program through a warranty on the UNiD Rod.
Fenske: More than 1,100 custom rods? That’s fantastic. What’s been the response from the surgeons working with this technology?

Richard Kienzle is the chief commercial and business development officer at Medicrea.
Fenske: In addition to providing the actual rods, how else are you collaborating with surgeons to enhance your solution?
Kienzle: The implant is just one piece of the puzzle here; UNiD represents a comprehensive service run through our UNiD Lab engineers who provide one-on-one dedicated support and value-add services to the entire process. Every surgery is an opportunity to improve surgeon experience and patient outcome. More and more spine surgeons are taking notice of Medicrea’s unique personalized approach. The UNiD program offers preoperative planning services; custom rod delivery based on the patient's plan; and a postoperative confirmation that the surgeon achieved the plan and restored the patient's unique sagittal balance.
Fenske: Since the rods are patient specific, what benefits are achieved during the procedure with regard to operating room time?
Kienzle: Surgeons were already “customizing” their rods but it happened during the operation using very basic bending tools, resulting in approximation in shape, loss of critical operating time, and even material damage—a known cause of postoperative implant failure. Essentially, surgeons were left to perform the last step in the implant manufacturing process without any support from device manufacturers to achieve their operative goals. With this hole in support, patient outcomes are clearly compromised. Medicrea stepped up to the plate to develop a patient-specific, outcome-focused solution with UNiD.
Fenske: It sounds like there is a combination of factors that all contribute to the success of the system that enables you to make the warranty offer. Would it be accurate to say there’s a natural synergy being created with each case between the company, the surgeon, the rods, the patient, the scans, the instruments, and the pre- and postoperative procedures?
Kienzle: There is certainly a synergy here that enables the UNiD program to simultaneously drive a value-based approach using personalized, data-driven medicine. What we have learned so far in pioneering this space in spine has convinced us that strategic surgical planning and analysis are key elements of a successful spinal surgery. Each time we collaborate with a surgeon, we are also benefiting by building a larger and larger data set that can be used to define the optimum strategy for the next individual patient. The data-driven UNiD program possesses an iterative learning capacity, so the more the surgeon uses it, the more data will be available to benchmark the next procedures. In essence, the system has a self-improvement mechanism, learning with each use.
Fenske: What do you foresee with regard to patient specific technology in the orthopedic space in the future? What’s coming five to ten years from now?
Kienzle: This is a busy and exciting time for us. We are continuing to expand the range of custom implants that can be generated through the UNiD program, while tracking a rapid uptake of the UNiD Rods in new and existing markets. We have already used the UNiD program to create custom 3D-printed interbody implants in Europe and expect to receive FDA clearance for the U.S. market before the end of 2017. We are also looking closely at using the program to develop new inventory control modalities that will significantly reduce the number of implants required to cover a typical spine surgery. The UNiD Lab will continue to be at the center of this expansion offering their comprehensive services and leading expertise to surgeons worldwide. As the custodians of personalized spine, we are hard at work ushering in a new era in patient care.
Fenske: Any other comments or thoughts you’d like to share?
Kienzle: Medicrea is pioneering a unique path that positions the company among the leading healthcare information technology firms and the next generation of outcome-driven device manufacturers. We are laser-focused on improving patient outcomes and providing system-wide cost reductions through a program of extensive clinical data compilation and mining, deep learning algorithms, and predictive analytics that culminates in precise and adaptive personalized spine care. In capturing a systems-based model for the iterative application of patient-specific spinal technology via UNiD, we are working to empirically answer the most difficult and complex clinical questions while providing strong, tangible value for healthcare shareholders benefiting patients, surgeons, hospitals, and payors in the process.
Because of this disruptive approach, we are neither restricted by nor economically dependent upon antiquated product revenue streams built upon clinical assumptions. We are also free of legacy manufacturing methods with their famously bloated distribution infrastructures. The 20th century medical device commercial concept that one size fits all and more is better does not live in our approach to spine. UNiD allows us to tackle tough, clinical questions and deploy sophisticated technology to validate patient outcomes, regardless of what the product technology or service solution need be.