Josh Cannon, Director of Global Healthcare Strategy, UPS06.30.17
The healthcare supply chain accounts for more than 40 percent of medical device costs, according to a report by McKinsey & Company. The UPS Pain in the (Supply) Chain survey shows that the biggest challenges to managing supply chain costs for healthcare manufacturers are rapid business growth, fluctuations in fuel and raw material costs, increasing regulations, and new market expansion. Add to that inventory control struggles and rampant industry consolidation, and you get a prescription for chaos.
To really get it right in the medical device supply chain, companies need tested and proven solutions that can be executed over and over for high-value, time-sensitive healthcare products. Companies must not only leverage logistics best practices, but also have the capabilities to take them to the next level in order to meet evolving, complex demands. In essence, medical device manufacturers should consider their supply chain the same way they look at new product development—as an area in need of continual innovation, efficiency, and forward thinking.
FSLs Improve Inventory Control
One of the biggest logistics challenges for medical device manufacturers is having excess inventory, which can be costly. A recent UPS analysis shows 73 percent of medical device manufacturers’ inventory is located out of their direct control [i.e., with sales representatives (trunk stock), in branch or field offices, or in hospital consignment]. This setup makes it extremely difficult to track and manage inventory and can result in product expiration, scrap, write-offs, and sub-optimal inventory turns.

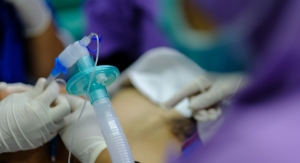
An employee inspects a medical device at UPS’s first facility, located in Swedesboro, N.J., to offer healthcare companies logistics services such as autoclave capabilities and surgical set replenishment. Image courtesy of UPS.
Collaborating with a third-party logistics provider (3PL) can help solve this challenge. Some 3PLs have field stocking location (FSL) networks that medical device manufacturers can tap into to place inventory within hours of medical facilities and surgery centers. By engaging a 3PL partnership, manufacturers are able to leverage an existing global network that is compliant with all regulations, enabling flexibility in the supply chain and gaining much-needed visibility into where inventory is located at all times. This asset-light supply chain model can drive big gains in efficiency and customer service—not to mention lowering inventory management costs.
New Technology Models
Lack of visibility in the supply chain is one of the top contributors to waste, product loss, and customer service failures. It could also lead to possible fines from regulatory groups with the enacting of Unique Device Identifier (UDI) guidelines, which have been phased in slowly since 2013 and will require full compliance by 2020.
Medical device manufacturers should look at technologies that give them full visibility across their supply chains, intelligence and insights about their key markets, and the ability to intervene when necessary.
One technology—radio frequency identification (RFID)—is evolving in sophistication. RFID tags once needed to be in very close proximity to the tag reader; they can now handle long-range scanning, allowing packages to move at greater speeds. In addition, once single-use tags are now reusable, and they can be read from smart phones. RFID technology is becoming an integral part of a smarter, more optimized supply chain. As this technology continues to evolve, it will become a more cost-effective solution for the medical device supply chain.
RFID is just one of several ways 3PLs can use track-and-trace capabilities to build out big data repositories—giving foresight into trends, delivering efficiencies, and pinpointing areas that need attention in the supply chain. Intervention technologies, such as GPS tracking, allow shippers to not only monitor shipments, but re-route them if needed.
Adopt Transformative Supply Chains
Looking at supply chains as a way to reduce costs, manage risks, and, ultimately, achieve business goals has to be mission critical within the industry. By treating the supply chain as a business asset, manufacturers can position themselves for flexibility and growth. Rethink inventory strategy, technology assets, and distribution network; embrace change and creativity; and be forward thinkers.
Innovative solutions lie at every handoff, turn, and liftoff point of the supply chain—if you know where to look. Agility and adaptability will define success for healthcare companies now more than ever before. Flexible and efficient supply chains that move with demand centers and cycles will become the new status quo. They will have lean inventory models that identify consumption clusters. They will have visibility solutions that allow logisticians to interpret situations and intervene when needed. They will have risk management and mitigation strategies incorporated end-to-end.
Efficiency Is the Bottom Line
High-value, critical devices such as orthopedic implants, cardiac pacemakers, insulin pumps, advanced surgical kits, and countless others have to reach new and existing markets in remarkably short timeframes. With new medical technologies improving patient outcomes and cutting-edge procedures requiring instruments being available at a moment’s notice, planning for a smart, efficient logistics network can be the difference between key enterprise gains or harsh business losses.
Aligning the supply chain to the strategic vision of an organization must be a key objective for consistent marketplace success. Today’s changing environment mandates that medical device companies rethink quality controls. Organizations that embrace change, shifting trends, and regulations and challenge their operational status quo will be better positioned for growth today and well into the future.
To really get it right in the medical device supply chain, companies need tested and proven solutions that can be executed over and over for high-value, time-sensitive healthcare products. Companies must not only leverage logistics best practices, but also have the capabilities to take them to the next level in order to meet evolving, complex demands. In essence, medical device manufacturers should consider their supply chain the same way they look at new product development—as an area in need of continual innovation, efficiency, and forward thinking.
FSLs Improve Inventory Control
One of the biggest logistics challenges for medical device manufacturers is having excess inventory, which can be costly. A recent UPS analysis shows 73 percent of medical device manufacturers’ inventory is located out of their direct control [i.e., with sales representatives (trunk stock), in branch or field offices, or in hospital consignment]. This setup makes it extremely difficult to track and manage inventory and can result in product expiration, scrap, write-offs, and sub-optimal inventory turns.

An employee inspects a medical device at UPS’s first facility, located in Swedesboro, N.J., to offer healthcare companies logistics services such as autoclave capabilities and surgical set replenishment. Image courtesy of UPS.
New Technology Models
Lack of visibility in the supply chain is one of the top contributors to waste, product loss, and customer service failures. It could also lead to possible fines from regulatory groups with the enacting of Unique Device Identifier (UDI) guidelines, which have been phased in slowly since 2013 and will require full compliance by 2020.
Medical device manufacturers should look at technologies that give them full visibility across their supply chains, intelligence and insights about their key markets, and the ability to intervene when necessary.
One technology—radio frequency identification (RFID)—is evolving in sophistication. RFID tags once needed to be in very close proximity to the tag reader; they can now handle long-range scanning, allowing packages to move at greater speeds. In addition, once single-use tags are now reusable, and they can be read from smart phones. RFID technology is becoming an integral part of a smarter, more optimized supply chain. As this technology continues to evolve, it will become a more cost-effective solution for the medical device supply chain.
RFID is just one of several ways 3PLs can use track-and-trace capabilities to build out big data repositories—giving foresight into trends, delivering efficiencies, and pinpointing areas that need attention in the supply chain. Intervention technologies, such as GPS tracking, allow shippers to not only monitor shipments, but re-route them if needed.
Adopt Transformative Supply Chains
Looking at supply chains as a way to reduce costs, manage risks, and, ultimately, achieve business goals has to be mission critical within the industry. By treating the supply chain as a business asset, manufacturers can position themselves for flexibility and growth. Rethink inventory strategy, technology assets, and distribution network; embrace change and creativity; and be forward thinkers.
Innovative solutions lie at every handoff, turn, and liftoff point of the supply chain—if you know where to look. Agility and adaptability will define success for healthcare companies now more than ever before. Flexible and efficient supply chains that move with demand centers and cycles will become the new status quo. They will have lean inventory models that identify consumption clusters. They will have visibility solutions that allow logisticians to interpret situations and intervene when needed. They will have risk management and mitigation strategies incorporated end-to-end.
Efficiency Is the Bottom Line
High-value, critical devices such as orthopedic implants, cardiac pacemakers, insulin pumps, advanced surgical kits, and countless others have to reach new and existing markets in remarkably short timeframes. With new medical technologies improving patient outcomes and cutting-edge procedures requiring instruments being available at a moment’s notice, planning for a smart, efficient logistics network can be the difference between key enterprise gains or harsh business losses.
Aligning the supply chain to the strategic vision of an organization must be a key objective for consistent marketplace success. Today’s changing environment mandates that medical device companies rethink quality controls. Organizations that embrace change, shifting trends, and regulations and challenge their operational status quo will be better positioned for growth today and well into the future.