William A. Hyman, Professor Emeritus of Biomedical Engineering, Texas A&M University, & Adjunct Professor of Biomedical Engineering, The Cooper Union06.15.18
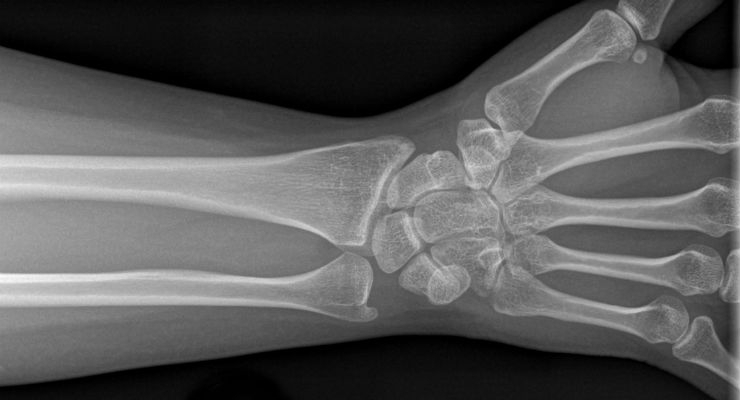
In the context of a De Novo review, as previously reported in ODT, of OsteoDetect’s wrist fracture analysis software, the FDA has newly defined “Radiological Computer Assisted Detection and Diagnosis Software” as:
“A radiological computer assisted detection and diagnostic software is an image processing device intended to aid in the detection, localization, and characterization of fracture, lesions, or other disease specific findings on acquired medical images (e.g. radiography, MR, CT). The device detects, identifies and characterizes findings based on features or information extracted from images, and provides information about the presence, location, and characteristics of the findings to the user. The analysis is intended to inform the primary diagnostic and patient management decisions that are made by the clinical user. The device is not intended as a replacement for a complete clinician's review or their clinical judgment that takes into account other relevant information from the image or patient history.”
This standalone software falls under the more general categories of Software as Medical Device (SaMD) and Clinical Decision Support (CDS). SaMD is any software that meets the definition of a medical device, but is not part of the function of another medical device. CDS is the subject of a draft regulation dated December 2017.
A De Novo finding brings with it Special Controls that allow such devices to be Class II, and subject to the 510(k) route to market. For this category of radiological software, the Special Controls includes several specific items for design verification and validation. In an abbreviated form, these require:
The Special Controls also stipulate labeling requirements including:
In the context of the specific device at issue, the FDA enumerated risks that are actually generic to diagnostic software. There are, in brief, false negatives and false positives even with the intended population, use for an excluded population or excluded hardware with correspondingly wrong results, and general failure which could lead to the absence, delay or incorrect results, and in turn, delayed or inaccurate patient diagnosis.
Not discussed in the De Novo order, but a general question for all SaMD and CDS, is how is the user of the software is supposed to deal with the results. This can range from “immediately believe and act on” to “deeply question every time.” Deeply question is particularly problematical for CDS based on machine learning using a very large dataset, in which case the user cannot possibly recreate the matching that the software provides. Whether “always use your professional judgment” has real-world meaning here is an open question.
“A radiological computer assisted detection and diagnostic software is an image processing device intended to aid in the detection, localization, and characterization of fracture, lesions, or other disease specific findings on acquired medical images (e.g. radiography, MR, CT). The device detects, identifies and characterizes findings based on features or information extracted from images, and provides information about the presence, location, and characteristics of the findings to the user. The analysis is intended to inform the primary diagnostic and patient management decisions that are made by the clinical user. The device is not intended as a replacement for a complete clinician's review or their clinical judgment that takes into account other relevant information from the image or patient history.”
This standalone software falls under the more general categories of Software as Medical Device (SaMD) and Clinical Decision Support (CDS). SaMD is any software that meets the definition of a medical device, but is not part of the function of another medical device. CDS is the subject of a draft regulation dated December 2017.
A De Novo finding brings with it Special Controls that allow such devices to be Class II, and subject to the 510(k) route to market. For this category of radiological software, the Special Controls includes several specific items for design verification and validation. In an abbreviated form, these require:
- A detailed description of the image analysis algorithm, including inputs and outputs, how the algorithm and output affects or relates to clinical practice or patient care, and any algorithm limitations.
- A detailed description of pre-specified performance testing protocols and dataset(s) used to assess whether the device will provide improved assisted-read detection and diagnostic performance as intended in the indicated user population(s). Performance testing is to include standalone test(s), side-by-side comparison(s), and/or a reader study, as applicable.
- Results from performance testing based on appropriate diagnostic accuracy measures. The test dataset must be representative of the typical patient population.
- Results from performance testing that demonstrate the device provides improved assisted read detection and/or diagnostic performance as intended in the indicated user population(s) when used in accordance with the instructions for use. The reader population must be comprised of the intended user population.
- Appropriate software documentation, including device hazard analysis, software requirements specification document, software design specification document, traceability analysis, system level test protocol, pass/fail criteria, testing results, and cybersecurity measures.
The Special Controls also stipulate labeling requirements including:
- A detailed description of the patient population for which the device is indicated for use
- A detailed description of the device instructions for use, including the intended reading protocol and how the user should interpret the device output
- A detailed description of the intended user, and any user training materials
- to ensure that the end user is fully aware of how to interpret and apply the device output
- A detailed description of the device inputs and outputs including compatible imaging hardware and imaging protocols
- Warnings, precautions, and limitations must include situations in which the device may fail or may not operate at its expected performance level
- A detailed summary of the performance testing
In the context of the specific device at issue, the FDA enumerated risks that are actually generic to diagnostic software. There are, in brief, false negatives and false positives even with the intended population, use for an excluded population or excluded hardware with correspondingly wrong results, and general failure which could lead to the absence, delay or incorrect results, and in turn, delayed or inaccurate patient diagnosis.
Not discussed in the De Novo order, but a general question for all SaMD and CDS, is how is the user of the software is supposed to deal with the results. This can range from “immediately believe and act on” to “deeply question every time.” Deeply question is particularly problematical for CDS based on machine learning using a very large dataset, in which case the user cannot possibly recreate the matching that the software provides. Whether “always use your professional judgment” has real-world meaning here is an open question.