Sean Fenske, Editor-in-Chief05.29.20
Spinal fusion is a well-established procedure in the orthopedic arena. Coupled with medtech’s tendencies to promote incremental innovation of technologies, it’s no wonder there hasn’t been significant advancement with regard to the recreation of the instrumentation for the surgery. Further, reservations regarding the durability and strength of single-use instrumentation have led to a slow adoption of the technology by many vendors.
There are exceptions, however, and Neo Medical is attempting to establish itself as one of those companies. The firm has developed and launched a complete solution for spinal fusion, leveraging disposable instrumentation and multi-functional tools. The result is a set that boasts substantially fewer pieces and helps to ensure infection control.
Orthopedic Design & Technology spoke with Vincent Lefauconnier, the CEO and founder of Neo Medical about his company’s innovation, and how it was being received within the surgical community. Lefauconnier holds a Master’s degree in biology and marketing and has 20 years of experience in marketing and sales in spine, which included stints with Medtronic and Stryker. He was previously the CCO of Vexim, which was acquired by Stryker.
Sean Fenske: What is Neo Medical’s spinal fusion offering?
Vincent Lefauconnier: Neo Medical developed a disruptive platform of Controlled Fixation solutions allowing for a more functional fusion in spinal surgery, respectful of patients’ unique spinal conditions.
With only five single-use sterile instruments, and only 14 versatile screws covering most thoracolumbar spine fusion indications in a universal approach, the unique proprietary features of Neo Medical’s solutions are designed to facilitate an anatomically neutral, balanced, and stable spine load bearing to achieve a more functional fusion. Neo Medical Controlled Fixation solutions also provide everyone involved in spinal surgery with an easier and more efficient experience, and offer additional value by limiting infections, removing re-sterilization needs, decluttering the OR, reducing reoperation rates and the associated costs, while ultimately putting patients, practitioners, payers, providers, and society in control. Beyond providing solutions tailored to patients’ needs, the Neo Medical value-based care approach participates in preserving the overall environmental sustainability of the broader healthcare system.
A full range of posterior highly innovative 3D-printed titanium cages has recently received CE marking and FDA approval. Along with Neo Medical Pedicle Screw System and through a single use, sterile, and universal instrument and implants offering, it reduces the inventory and instrumentation needed while providing more options to optimize patient outcomes and more value to most stakeholder involved in spinal surgery.
Fenske: How does it differ from other spinal fusion solutions available today?
Lefauconnier: Spinal fusion surgery is one of the most common options for back-related pain, and over the years, it has given crucial relief to many patients requiring spine treatment. However, its results have been widely debated since outcomes do not always meet surgeon and patient expectations. There are many reasons and factors to that. Among them, postoperative pain, implant failures or loosening, Adjacent Segment Disease, and infections all have the potential to trigger secondary surgery, of which, the efficacy is further debated. While pedicle screws and associated instruments were historically designed for the treatment of spinal deformities, their use has gradually been expanded to degenerative indications over the past 30 years. To this day, however, the medtech industry has proposed mostly incremental innovations.
Using the same product technology and techniques for different indications and etiologies has often meant placing instruments and implants too forcefully on patients’ spines, which can be described as forced fixation. The direct consequence is a stress overload on patients’ spines with a domino effect as non-physiological forces and unwanted strain gradually change the natural biomechanics of the spine and can generate a three-dimensional spinal imbalance. This potential breakdown of the anatomical and biomechanical balance of the patient’s spine may trigger a chain reaction that can result in a revision surgery.
We believe spinal fusion surgery is currently at a crossroad. Faced with the finding that the stress factors potentially created by current fixation devices are much greater on the anatomy than anticipated, we think we need to embark on a quest for more functional and physiological options.
At Neo Medical, we recognize too much is at stake to be satisfied with today’s standard of care and incremental changes only. While we appreciate multiple reasons can lead to suboptimal results in spine, it is our intention to help address the situation by driving surgical techniques with the development of a new disruptive modular technology for controlled fixation, more respectful of patients’ unique spinal conditions.
Fenske: How does your technology enable such a substantial reduction of instruments and components compared to more traditional spinal fusion solutions?
Lefauconnier: The answer lies in the innovative concept of controlled fixation and the approach we took right from the beginning of our product development.
By starting from a blank page, basing our approach on science and published data, we have looked at and optimized every step of the surgery to give back the full control into the surgeon hands. With the objective to ensure the right correction, when required, and provide a fixation that can be perfectly adapted for every indication and most patients’ anatomy, we developed unique patented technologies resulting in a light, modular, and universal platform now replacing current product offering.
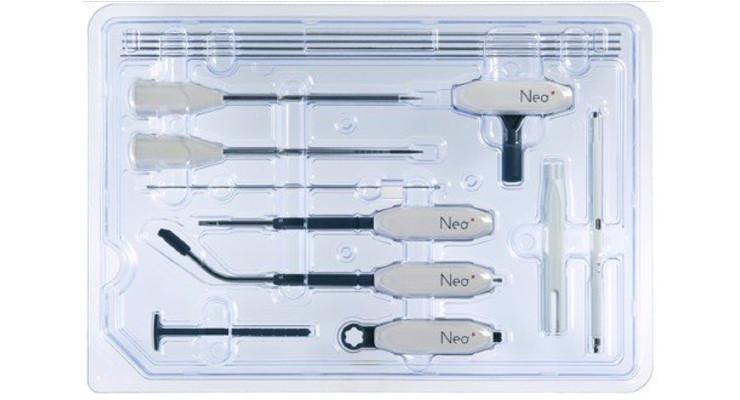
With only five single-use sterile instruments, and only 14 versatile screws covering most thoracolumbar spine fusion indications in a universal approach, the unique proprietary features of Neo Medical’s solutions are designed to facilitate an anatomically neutral, balanced, and stable spine load bearing to achieve a more functional fusion.
Instead of three to five trays of instruments for open surgery and about the same for minimally invasive procedures, we offer five multi-functional instruments for universal indications (tumor/trauma/degenerative/deformity) and pathologies that can be used in both open and MIS approaches. In addition, instead of multiple styles of screws (up to hundreds of different implants in current systems), we only need a single style with all of the features built into one (14 screws currently, with the plan to increase it to 25 in the coming months).
Fenske: How do the changes to technique and technology benefit surgeons? What are the savings with your solution?
Lefauconnier: It is a whole new world! With only five universal and multi-functional instruments, covering most spine indications with unique proprietary features, the system is so streamlined it can create massive efficiencies by making the surgery potentially faster, more flexible, intuitive, and safer.
As a matter of fact, a study conducted in Germany on 150 patients (12 months follow-up, data on file) is showing significantly less time in surgery, a rate of infection of only 2 percent (usually literature are showing rates of 4 to 13 percent in spinal surgery), and rate of implant failure of only 1.5 percent (screw loosening, no implant breakages).
The drastically reduced number of tools, unique Swiss manufacturing approach, and the single-use/sterile packaging also means a massive reduction by 75 percent of the environmental footprint versus legacy reusable systems (study published in January 2020).
But Neo fusion solutions do not only benefit surgeons; they also make the work of anyone involved in spinal surgery easier and more efficient, and provide value by limiting infections, potentially reducing reoperation rates and associated costs.

With only five universal and multi-functional instruments, covering most spine indications with unique proprietary features, the system is so streamlined it can create massive efficiencies by making the surgery potentially faster, more flexible, intuitive, and safer.
Fenske: What’s the response from surgeons who need to adjust their technique with your technology? Do you encounter resistance from them on this aspect?
Lefauconnier: So far, surgeon feedback has been excellent, as they immediately understand the benefits our controlled-fixation approach can bring to their procedures and patients, as well as the slight adjustments to their surgical technique.
To date, we have more than 250 centers using our technologies and growing fast, while 90 percent of our procedures are coming from recurrent users, showing a high level of satisfaction as our technologies provide benefits to their patients, institutions, and also themselves.
We recently put in place a new digital approach to our training platform called SENSE (Spine Expert Network for Science and Education) and we are getting an increasing number of requests to participate in our programs. From what we see, not only surgeons, but nearly everyone involved in spinal surgery, were waiting for such a solution to address current challenges in spinal surgery.
Fenske: What have you found is the environmental impact with single-use instruments compared to reusable?
Lefauconnier: The worldwide increasing wealth and increased life expectancy of humans has led to an increase in the number of medical procedures and surgeries. However, surgeries are complex medical procedures that contribute to a significant share of the total environmental impact of the healthcare system. Among other important sources of environmental impacts from surgeries, material consumption due to required instrumentation accounts for up to 65 percent of greenhouse gas emissions.
We have recently seen the publication of a study investigating how the Neo Medical disposable solution and reusable surgery instrument sets for lumbar fusion surgeries contribute to the environmental impact and which system is more advantageous for the environment.
To compare the environmental impact of these different configurations, a comparative Life Cycle Assessment (LCA) was performed. One of the key findings was the selected cleaning and sterilization process for reusable instruments is responsible for up to 90 percent of the greenhouse gas emissions and decides which system is advantageous from an environmental perspective. This study has shown a 75 percent reduction in the overall environmental footprint per surgery of our technology versus one of the legacy reusable systems. More specifically, on climate change, energy consumption, and resource depletion, the impact went up to 85 percent improvement in favor of the Neo Medical system.
Fenske: What’s ahead for Neo Medical?
Lefauconnier: We have recently closed a 13.2 million CHFs ($13.4 million U.S.) financing round to finance the growth and expansion of the company in priority markets worldwide. Our new unique cage platform is now going to be fully released in our different markets and several new technologies will hit the markets in 2020 and 2021. We also have several studies further establishing our value proposition to be released in the coming months.
Fenske: Do you have any additional thoughts or comments?
Lefauconnier: In these exceptional times we are living, Neo Medical is well positioned to provide our partners solutions we believe have the potential to radically improve not only the way we are treating patients, but also the way we are doing business. We are at a pivotal time when, beyond providing solutions tailored to patients’ needs, the Neo Medical value-based care approach is leading the way to preserve and improve the overall environmental and economical sustainability of the broader healthcare system.
There are exceptions, however, and Neo Medical is attempting to establish itself as one of those companies. The firm has developed and launched a complete solution for spinal fusion, leveraging disposable instrumentation and multi-functional tools. The result is a set that boasts substantially fewer pieces and helps to ensure infection control.
Orthopedic Design & Technology spoke with Vincent Lefauconnier, the CEO and founder of Neo Medical about his company’s innovation, and how it was being received within the surgical community. Lefauconnier holds a Master’s degree in biology and marketing and has 20 years of experience in marketing and sales in spine, which included stints with Medtronic and Stryker. He was previously the CCO of Vexim, which was acquired by Stryker.
Sean Fenske: What is Neo Medical’s spinal fusion offering?
Vincent Lefauconnier: Neo Medical developed a disruptive platform of Controlled Fixation solutions allowing for a more functional fusion in spinal surgery, respectful of patients’ unique spinal conditions.
With only five single-use sterile instruments, and only 14 versatile screws covering most thoracolumbar spine fusion indications in a universal approach, the unique proprietary features of Neo Medical’s solutions are designed to facilitate an anatomically neutral, balanced, and stable spine load bearing to achieve a more functional fusion. Neo Medical Controlled Fixation solutions also provide everyone involved in spinal surgery with an easier and more efficient experience, and offer additional value by limiting infections, removing re-sterilization needs, decluttering the OR, reducing reoperation rates and the associated costs, while ultimately putting patients, practitioners, payers, providers, and society in control. Beyond providing solutions tailored to patients’ needs, the Neo Medical value-based care approach participates in preserving the overall environmental sustainability of the broader healthcare system.
A full range of posterior highly innovative 3D-printed titanium cages has recently received CE marking and FDA approval. Along with Neo Medical Pedicle Screw System and through a single use, sterile, and universal instrument and implants offering, it reduces the inventory and instrumentation needed while providing more options to optimize patient outcomes and more value to most stakeholder involved in spinal surgery.
Fenske: How does it differ from other spinal fusion solutions available today?
Lefauconnier: Spinal fusion surgery is one of the most common options for back-related pain, and over the years, it has given crucial relief to many patients requiring spine treatment. However, its results have been widely debated since outcomes do not always meet surgeon and patient expectations. There are many reasons and factors to that. Among them, postoperative pain, implant failures or loosening, Adjacent Segment Disease, and infections all have the potential to trigger secondary surgery, of which, the efficacy is further debated. While pedicle screws and associated instruments were historically designed for the treatment of spinal deformities, their use has gradually been expanded to degenerative indications over the past 30 years. To this day, however, the medtech industry has proposed mostly incremental innovations.
Using the same product technology and techniques for different indications and etiologies has often meant placing instruments and implants too forcefully on patients’ spines, which can be described as forced fixation. The direct consequence is a stress overload on patients’ spines with a domino effect as non-physiological forces and unwanted strain gradually change the natural biomechanics of the spine and can generate a three-dimensional spinal imbalance. This potential breakdown of the anatomical and biomechanical balance of the patient’s spine may trigger a chain reaction that can result in a revision surgery.
We believe spinal fusion surgery is currently at a crossroad. Faced with the finding that the stress factors potentially created by current fixation devices are much greater on the anatomy than anticipated, we think we need to embark on a quest for more functional and physiological options.
At Neo Medical, we recognize too much is at stake to be satisfied with today’s standard of care and incremental changes only. While we appreciate multiple reasons can lead to suboptimal results in spine, it is our intention to help address the situation by driving surgical techniques with the development of a new disruptive modular technology for controlled fixation, more respectful of patients’ unique spinal conditions.
Fenske: How does your technology enable such a substantial reduction of instruments and components compared to more traditional spinal fusion solutions?
Lefauconnier: The answer lies in the innovative concept of controlled fixation and the approach we took right from the beginning of our product development.
By starting from a blank page, basing our approach on science and published data, we have looked at and optimized every step of the surgery to give back the full control into the surgeon hands. With the objective to ensure the right correction, when required, and provide a fixation that can be perfectly adapted for every indication and most patients’ anatomy, we developed unique patented technologies resulting in a light, modular, and universal platform now replacing current product offering.

With only five single-use sterile instruments, and only 14 versatile screws covering most thoracolumbar spine fusion indications in a universal approach, the unique proprietary features of Neo Medical’s solutions are designed to facilitate an anatomically neutral, balanced, and stable spine load bearing to achieve a more functional fusion.
Instead of three to five trays of instruments for open surgery and about the same for minimally invasive procedures, we offer five multi-functional instruments for universal indications (tumor/trauma/degenerative/deformity) and pathologies that can be used in both open and MIS approaches. In addition, instead of multiple styles of screws (up to hundreds of different implants in current systems), we only need a single style with all of the features built into one (14 screws currently, with the plan to increase it to 25 in the coming months).
Fenske: How do the changes to technique and technology benefit surgeons? What are the savings with your solution?
Lefauconnier: It is a whole new world! With only five universal and multi-functional instruments, covering most spine indications with unique proprietary features, the system is so streamlined it can create massive efficiencies by making the surgery potentially faster, more flexible, intuitive, and safer.
As a matter of fact, a study conducted in Germany on 150 patients (12 months follow-up, data on file) is showing significantly less time in surgery, a rate of infection of only 2 percent (usually literature are showing rates of 4 to 13 percent in spinal surgery), and rate of implant failure of only 1.5 percent (screw loosening, no implant breakages).
The drastically reduced number of tools, unique Swiss manufacturing approach, and the single-use/sterile packaging also means a massive reduction by 75 percent of the environmental footprint versus legacy reusable systems (study published in January 2020).
But Neo fusion solutions do not only benefit surgeons; they also make the work of anyone involved in spinal surgery easier and more efficient, and provide value by limiting infections, potentially reducing reoperation rates and associated costs.

With only five universal and multi-functional instruments, covering most spine indications with unique proprietary features, the system is so streamlined it can create massive efficiencies by making the surgery potentially faster, more flexible, intuitive, and safer.
Lefauconnier: So far, surgeon feedback has been excellent, as they immediately understand the benefits our controlled-fixation approach can bring to their procedures and patients, as well as the slight adjustments to their surgical technique.
To date, we have more than 250 centers using our technologies and growing fast, while 90 percent of our procedures are coming from recurrent users, showing a high level of satisfaction as our technologies provide benefits to their patients, institutions, and also themselves.
We recently put in place a new digital approach to our training platform called SENSE (Spine Expert Network for Science and Education) and we are getting an increasing number of requests to participate in our programs. From what we see, not only surgeons, but nearly everyone involved in spinal surgery, were waiting for such a solution to address current challenges in spinal surgery.
Fenske: What have you found is the environmental impact with single-use instruments compared to reusable?
Lefauconnier: The worldwide increasing wealth and increased life expectancy of humans has led to an increase in the number of medical procedures and surgeries. However, surgeries are complex medical procedures that contribute to a significant share of the total environmental impact of the healthcare system. Among other important sources of environmental impacts from surgeries, material consumption due to required instrumentation accounts for up to 65 percent of greenhouse gas emissions.
We have recently seen the publication of a study investigating how the Neo Medical disposable solution and reusable surgery instrument sets for lumbar fusion surgeries contribute to the environmental impact and which system is more advantageous for the environment.
To compare the environmental impact of these different configurations, a comparative Life Cycle Assessment (LCA) was performed. One of the key findings was the selected cleaning and sterilization process for reusable instruments is responsible for up to 90 percent of the greenhouse gas emissions and decides which system is advantageous from an environmental perspective. This study has shown a 75 percent reduction in the overall environmental footprint per surgery of our technology versus one of the legacy reusable systems. More specifically, on climate change, energy consumption, and resource depletion, the impact went up to 85 percent improvement in favor of the Neo Medical system.
Fenske: What’s ahead for Neo Medical?
Lefauconnier: We have recently closed a 13.2 million CHFs ($13.4 million U.S.) financing round to finance the growth and expansion of the company in priority markets worldwide. Our new unique cage platform is now going to be fully released in our different markets and several new technologies will hit the markets in 2020 and 2021. We also have several studies further establishing our value proposition to be released in the coming months.
Fenske: Do you have any additional thoughts or comments?
Lefauconnier: In these exceptional times we are living, Neo Medical is well positioned to provide our partners solutions we believe have the potential to radically improve not only the way we are treating patients, but also the way we are doing business. We are at a pivotal time when, beyond providing solutions tailored to patients’ needs, the Neo Medical value-based care approach is leading the way to preserve and improve the overall environmental and economical sustainability of the broader healthcare system.