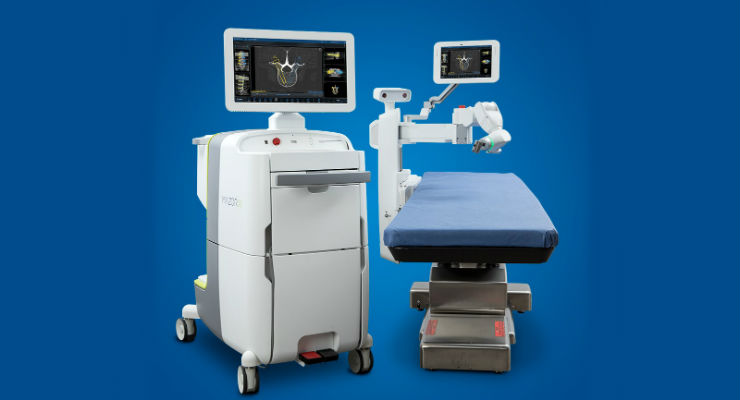
Richard V. Chua, M.D., FAANS, FACS, Northwest NeuroSpecialists PLLC and professor, Department of Neurosurgery, University of Arizona College of Medicine09.24.20
Transforaminal lumbar interbody fusion (TLIF), the most common technique used for lumbar fusion, can treat various common degenerative spine conditions including degenerative disc disease, spinal stenosis, recurrent herniated discs, and low-grade spondylolisthesis. A key factor in the success of a TLIF, whether performed as an open or minimally invasive surgery (MIS), is the accuracy and precision of pedicle screw placement as determined by pre-surgical imaging and pre-operative planning. Precise and accurate pedicle screw placement can reduce the risk of complications related to misplaced instrumentation that can lead to nerve injury and the need for subsequent surgical procedures.
The Benefits of Robot-Assisted TLIF
A key benefit of integrating robotic systems into TLIF procedures is they can improve surgical accuracy. Computerized pre-operative planning provides a 3D assessment of spinal anatomy that allows a variety of screw and implant sizes to be tested virtually, enabling development of a precise and accurate surgical plan. Patient-specific construct simulation allows true alignment of vertebral bodies to show consistent level-to-level view of the anatomy; enables screw head alignment, rod and construct optimization, and planning of long constructs and challenging small pedicles; and supports cage planning simulations and osteotomy planning. The use of 3D analytical software also provides automatic calculations of common spinal measurements and allows alignment simulations. Robotic guidance during the procedure helps to ensure the surgical plan is executed accurately and precisely, and image-guidance techniques allow real-time imaging to confirm the pedicle screw has been properly placed. Another benefit to robotic systems is they reduce patient and surgical personnel intra-operative X-ray exposure. Fewer X-rays are needed during the procedure when robot-assisted, and image-guidance techniques are combined.
Integration of Robotics Into Image-Guided MIS-TLIF Procedures
Robotic systems that combine state-of-the-art technologies can reduce procedure time, improve efficiencies, and create a more efficient and standardized surgical solution. Importantly, these systems can be readily integrated into open and minimally invasive techniques for instrumented fusions. Minimally invasive TLIF is a well-accepted procedure with excellent short-term and long-term outcomes, and has been shown to provide comparable or improved clinical outcomes compared with open posterior interbody fusion techniques, with significantly less blood loss, hospital stay and complications.1 Robotics have the potential to augment these outcomes, and a recent study found that robot-assisted MIS-TLIF using an O-arm navigation resulted in less intraoperative blood loss, shorter bed rest time and hospitalization stay, and more precise screw placement (p<0.05 for each) compared with conventional TLIF, with no difference in visual analog scale or Oswetry disability index scores between the two approaches at the last follow-up visit.2 Thus, robotic technology can enhance the success of MIS-TLIF.
In the context of MIS-TLIF, the planning software and robotic arm guidance allow surgeons to use 3D imaging to guide pedicle screw placement. Additionally, 3D image-guided navigational techniques that integrate multiple X-ray images to create 3D spinal images during surgery enable more precise and accurate placement of screws and other instruments into the spine.3 Significantly, incision size and amount of soft tissue exposed to reach the spine can be reduced with improved precision of the incision as well as the pedicle screw placement, as the surgeon can dissect to the desired location without requiring additional space to adjust the final screw placement. A smaller incision and more precise pedicle screw placement may help to reduce blood loss during the surgery, risk of infection, and post-operative pain; shorten hospitalization and recovery time; and reduce post-operative pain medication use.
Robotic systems may also improve efficiencies within the operating room (OR). Precision pre-planning can determine the exact sizes and necessary instrument configuration for the procedure, thus reducing the number of instrument sets, trays, and equipment brought to the OR. In this regard, robotic systems may improve supply chain issues.
Future applications of robotic technologies in MIS-TLIF may include pre-operative planning followed by robotic execution of decompression techniques, use of drills, interbody cage placement, and deformity correction. As robotic technology continues to improve, developing safe workflows that integrate robotics with currently well-established techniques should continue to improve patient outcomes.
Case Study of a Robot-Assisted MIS-TLIF Procedure
A 69-year old man with prior history of coronary artery disease, valvular heart disease, high blood pressure, and a cardiac arrhythmia requiring a pacemaker, was seen for an approximately two-year history of progressively worsening back pain. The pain travelled down the right leg into the great toe and was associated with occasional numbness and tingling in the right foot. His primary care physician prescribed medications and physical therapy, which were not helpful. He was then seen and treated with a spinal epidural steroid injection at a pain clinic, which likewise provided little benefit. Taking high doses of Ibuprofen, and occasional prescription opiate medications, the man was unable to walk his dog or go on hikes with his wife, and had trouble sitting, standing, or walking for more than 10 to 15 minutes.
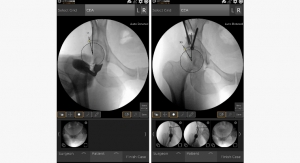
This patient had X-rays and a CT scan (he was unable to get an MRI scan) and was scheduled to see a neurosurgeon. He had mild weakness in his right leg and walked with a noticeable limp. Review of his X-rays and CT scan identified a cyst from the L4-5 facet joint, which was causing pressure on the nerves of the spine, particularly on the right side. In addition, the X-rays showed instability, with movement of the L4 bone on top of the L5 bone.
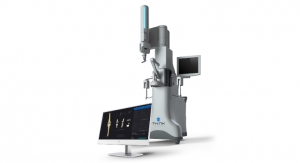
The patient’s symptoms correlated well with the examination and the CT findings. He was recommended to undergo a robot-assisted, image-guided, minimally invasive L4-5 removal of the synovial cyst and TLIF using instrumentation. He underwent the surgery using the Mazor X Stealth Edition, which took approximately two hours. He was walking within a few hours and was released from the hospital the following morning. He was completely relieved of his pain and took pain medicine sparingly for about five days. He was seen for a follow-up visit at three weeks, at which time he was walking about three miles a day. At his one-year checkup, he continued to do well without any significant pain, was back to his usual activity level including hiking, and his X-rays and CT scan showed a successful fusion.
The Promise of Ongoing Innovation
While robotic technology is enabling improved workflows and outcomes for patients undergoing MIS-TLIF, ongoing innovation should provide additional benefits. With sophistication and experience with image-guided platforms, Medtronic Stealth is considered to be an industry leader in image guidance, and Mazor was a robotics-focused company dedicated to development of novel spine surgery applications prior to its acquisition by Medtronic. Combined with Medtronic’s track record of leadership in neurosurgical and spinal instruments, drills and implants, the Mazor X Stealth Edition platform is enabling innovative approaches to TLIF while providing a foundation for continued advancement.
Future applications of the Mazor X Stealth Edition are expected to provide advantages such as enhanced segmentation software that provide new solutions for surgical guidance. Additionally, the current platform is expected to soon be able to plan and execute other steps of the operation (not just guiding screw placement), including decompression, placement of interbody spacer/graft, and accurate and pre-planned correction of deformity. Future advances also include the potential to incorporate artificial intelligence that has the potential to further improve the accuracy, safety, and efficiency of robot-assisted MIS-TLIF. Surgeons who adopt today’s most cutting-edge robotic technologies will be well positioned to benefit from future advances and to offer today’s and tomorrow’s patients the best outcomes possible.
References
1 Karikari IO and Isaacs RE. Minimally invasive transforaminal lumbar interbody fusion: a review of techniques and outcomes. Spine. 2010;35(26 Suppl):S294-301.
The Benefits of Robot-Assisted TLIF
A key benefit of integrating robotic systems into TLIF procedures is they can improve surgical accuracy. Computerized pre-operative planning provides a 3D assessment of spinal anatomy that allows a variety of screw and implant sizes to be tested virtually, enabling development of a precise and accurate surgical plan. Patient-specific construct simulation allows true alignment of vertebral bodies to show consistent level-to-level view of the anatomy; enables screw head alignment, rod and construct optimization, and planning of long constructs and challenging small pedicles; and supports cage planning simulations and osteotomy planning. The use of 3D analytical software also provides automatic calculations of common spinal measurements and allows alignment simulations. Robotic guidance during the procedure helps to ensure the surgical plan is executed accurately and precisely, and image-guidance techniques allow real-time imaging to confirm the pedicle screw has been properly placed. Another benefit to robotic systems is they reduce patient and surgical personnel intra-operative X-ray exposure. Fewer X-rays are needed during the procedure when robot-assisted, and image-guidance techniques are combined.
Integration of Robotics Into Image-Guided MIS-TLIF Procedures
Robotic systems that combine state-of-the-art technologies can reduce procedure time, improve efficiencies, and create a more efficient and standardized surgical solution. Importantly, these systems can be readily integrated into open and minimally invasive techniques for instrumented fusions. Minimally invasive TLIF is a well-accepted procedure with excellent short-term and long-term outcomes, and has been shown to provide comparable or improved clinical outcomes compared with open posterior interbody fusion techniques, with significantly less blood loss, hospital stay and complications.1 Robotics have the potential to augment these outcomes, and a recent study found that robot-assisted MIS-TLIF using an O-arm navigation resulted in less intraoperative blood loss, shorter bed rest time and hospitalization stay, and more precise screw placement (p<0.05 for each) compared with conventional TLIF, with no difference in visual analog scale or Oswetry disability index scores between the two approaches at the last follow-up visit.2 Thus, robotic technology can enhance the success of MIS-TLIF.
In the context of MIS-TLIF, the planning software and robotic arm guidance allow surgeons to use 3D imaging to guide pedicle screw placement. Additionally, 3D image-guided navigational techniques that integrate multiple X-ray images to create 3D spinal images during surgery enable more precise and accurate placement of screws and other instruments into the spine.3 Significantly, incision size and amount of soft tissue exposed to reach the spine can be reduced with improved precision of the incision as well as the pedicle screw placement, as the surgeon can dissect to the desired location without requiring additional space to adjust the final screw placement. A smaller incision and more precise pedicle screw placement may help to reduce blood loss during the surgery, risk of infection, and post-operative pain; shorten hospitalization and recovery time; and reduce post-operative pain medication use.
Robotic systems may also improve efficiencies within the operating room (OR). Precision pre-planning can determine the exact sizes and necessary instrument configuration for the procedure, thus reducing the number of instrument sets, trays, and equipment brought to the OR. In this regard, robotic systems may improve supply chain issues.
Future applications of robotic technologies in MIS-TLIF may include pre-operative planning followed by robotic execution of decompression techniques, use of drills, interbody cage placement, and deformity correction. As robotic technology continues to improve, developing safe workflows that integrate robotics with currently well-established techniques should continue to improve patient outcomes.
Case Study of a Robot-Assisted MIS-TLIF Procedure
A 69-year old man with prior history of coronary artery disease, valvular heart disease, high blood pressure, and a cardiac arrhythmia requiring a pacemaker, was seen for an approximately two-year history of progressively worsening back pain. The pain travelled down the right leg into the great toe and was associated with occasional numbness and tingling in the right foot. His primary care physician prescribed medications and physical therapy, which were not helpful. He was then seen and treated with a spinal epidural steroid injection at a pain clinic, which likewise provided little benefit. Taking high doses of Ibuprofen, and occasional prescription opiate medications, the man was unable to walk his dog or go on hikes with his wife, and had trouble sitting, standing, or walking for more than 10 to 15 minutes.
This patient had X-rays and a CT scan (he was unable to get an MRI scan) and was scheduled to see a neurosurgeon. He had mild weakness in his right leg and walked with a noticeable limp. Review of his X-rays and CT scan identified a cyst from the L4-5 facet joint, which was causing pressure on the nerves of the spine, particularly on the right side. In addition, the X-rays showed instability, with movement of the L4 bone on top of the L5 bone.
The patient’s symptoms correlated well with the examination and the CT findings. He was recommended to undergo a robot-assisted, image-guided, minimally invasive L4-5 removal of the synovial cyst and TLIF using instrumentation. He underwent the surgery using the Mazor X Stealth Edition, which took approximately two hours. He was walking within a few hours and was released from the hospital the following morning. He was completely relieved of his pain and took pain medicine sparingly for about five days. He was seen for a follow-up visit at three weeks, at which time he was walking about three miles a day. At his one-year checkup, he continued to do well without any significant pain, was back to his usual activity level including hiking, and his X-rays and CT scan showed a successful fusion.
The Promise of Ongoing Innovation
While robotic technology is enabling improved workflows and outcomes for patients undergoing MIS-TLIF, ongoing innovation should provide additional benefits. With sophistication and experience with image-guided platforms, Medtronic Stealth is considered to be an industry leader in image guidance, and Mazor was a robotics-focused company dedicated to development of novel spine surgery applications prior to its acquisition by Medtronic. Combined with Medtronic’s track record of leadership in neurosurgical and spinal instruments, drills and implants, the Mazor X Stealth Edition platform is enabling innovative approaches to TLIF while providing a foundation for continued advancement.
Future applications of the Mazor X Stealth Edition are expected to provide advantages such as enhanced segmentation software that provide new solutions for surgical guidance. Additionally, the current platform is expected to soon be able to plan and execute other steps of the operation (not just guiding screw placement), including decompression, placement of interbody spacer/graft, and accurate and pre-planned correction of deformity. Future advances also include the potential to incorporate artificial intelligence that has the potential to further improve the accuracy, safety, and efficiency of robot-assisted MIS-TLIF. Surgeons who adopt today’s most cutting-edge robotic technologies will be well positioned to benefit from future advances and to offer today’s and tomorrow’s patients the best outcomes possible.
References
1 Karikari IO and Isaacs RE. Minimally invasive transforaminal lumbar interbody fusion: a review of techniques and outcomes. Spine. 2010;35(26 Suppl):S294-301.
2 Peng P, Chen K, Chen H, et al. Comparison of O-arm navigation and microscope-assisted minimally invasive transforaminal lumbar interbody fusion and conventional transforaminal lumbar interbody fusion for the treatment of lumbar isthmic spondylolisthesis. J Orthoped Trans. 2020:107-112. https://doi.org/10.1016/j.jot.2019.10.001
3 Kochanski RB, Lombardi JM, Laratta JL, et al. Image-guided navigation and robotics in spine surgery. Neurosurgery. 2019;84:1179-1189.