Videos
Magnetic Fields Wipe Out Bacteria from Artificial Joints
Magnetic Fields Wipe Out Bacteria from Artificial Joints
Periprosthetic joint infections are a devastating complication and a significant financial burden to the healthcare system.
By UT Southwestern Medical Center08.10.17
A short exposure to an alternating magnetic field might someday replace multiple surgeries and weeks of IV antibiotics as treatment for stubborn infections on artificial joints, new research suggests.
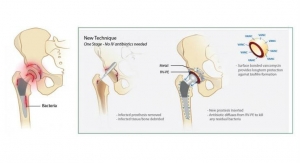
Researchers at UT Southwestern Medical Center have shown that high-frequency alternating magnetic fields—the same principle used in induction cooktops—can be used to destroy bacteria that are encased in a slimy “biofilm” growing on a metal surface. The biofilm is a collection of microorganisms that stick to each other as well as to various surfaces. This aggregate of organisms and other substances that surround them makes it difficult for both drugs and immune cells to reach the bacteria.
Technology developed by Dr. Rajiv Chopra and Dr. David Greenberg uses this method to heat the surface of prosthetic metal joints and destroy bacterial biofilms. Their work appears in Scientific Reports.
When a metal is in the presence of alternating magnetic fields (AMFs), electrical currents are produced on the metal, which generate heat. If the direction of the magnetic fields is rapidly switched back and forth (i.e., high frequency) then electrical currents only flow along the outer edge of the metal, which is where the biofilm is.
“That was the lightbulb in the conversation,” said Dr. Chopra, Associate Professor of Radiology and with the Advanced Imaging Research Center. “You have a pathogen that can’t be treated with conventional drugs. You have a physical effect—heating on the surface of a metal—that’s often a complication for imaging technologies such as MRI. We’ve taken two things that are problems and, by putting them together, we’ve turned up a solution.”
Dr. Greenberg, Associate Professor of Internal Medicine and of Microbiology, said biofilm-related infections are one of the most serious complications of knee and hip replacements, often requiring multiple surgeries. “We were looking for better ways to target and treat biofilms. Our idea was to put a coil around the joint and run a current through it to create alternating magnetic fields. Human tissue isn’t conductive but metal is, so only the implant would heat up.”
Using prosthetic joint models, the researchers showed that heating the metal surface via AMFs destroyed biofilm and killed bacteria. They tested several species of bacteria that create biofilm on artificial joints, and the process worked with each type.
Another key finding of their work is that AMF treatment increased the effectiveness of antibiotic treatment. “Think about heat as a drug for a minute,” said Dr. Greenberg. “Sometimes in infectious diseases we want to know whether two drugs are synergistic, meaning one plus one equals four, which is the case with AMF and antibiotics. That’s important because if treatments are synergistic, you can use lower concentrations of each, which has implications for safety as well as cost.”
Mouse-model safety tests indicated that high-power, short-duration doses of AMF minimize heat damage to adjoining tissue. High power achieves a target temperature on the surface of the metal device quickly, before there is much time for heat to accumulate in surrounding tissues.
Each year, more than 1 million knee and hip replacements are performed in the United States, and the numbers are expected to rise dramatically over the next decade with rising rates of obesity and an aging population.
While prosthetic joint infection rates are low, when they do occur they are exceptionally difficult to treat. Current treatment for an infection in a prosthetic knee involves removal of the biofilm-covered joint, placement of a temporary spacer, a six-week course of intravenous antibiotics, and then a second knee-replacement surgery that’s more complex to perform than the original surgery.
Patients are unable to take part in normal activities for months.
“Periprosthetic joint infections are a devastating complication to patients and are a significant financial burden to our health care system,” said orthopaedic surgeon Dr. Kenneth Estrera, an Assistant Professor at UT Southwestern. “The research by Dr. Greenberg and Dr. Chopra may lead to a solution to the most challenging problem in joint arthroplasty surgery.”
Future work includes more animal-model tests of safety and effectiveness.
Other UT Southwestern researchers who contributed to this project are Sumbul Shaikh, research assistant; Yonatan Chatzinoff, biomedical engineering specialist; Dr. Imalka Munaweera, post-doctoral researcher; Dr. Bingbing Cheng, post-doctoral researcher; Dr. Seth M. Daly, post-doctoral researcher; Dr. Yin Xi, Assistant Professor of Radiology and of the Department of Clinical Sciences; Chenchen Bing, research associate; and Dr. Dennis Burns, Professor of Pathology and holder of the Jane B. and Edwin P. Jenevein, M.D. Chair in Pathology.
Related Searches: