Regulatory Perspectives
- All Departments
- Asia News
- Best Practices
- Clinical Trials
- Data Watch
- Design Viewpoint
- Financial News
- Financial Perspectives
- Global Perspectives
- Industry News
- International Markets
- Market Snapshot
- New Technology Update
- News Front
- Orthopedic Insights
- Outsourcing Efficiencies
- Patent Law
- People News
- Process Improvement
- Product Announcements
- Quality Perspectives
- Regulatory Perspectives
- Reimbursement Roadmap
- Risk Management
- Sector Spotlight
- Technology Profile
- The Last Word
- Washington Roundup
-
Biologics

CMC Considerations for Medical Device-Led Combination Products
Creating antibiotic-loaded orthopedic combination products demands meticulous yet distinct CMC considerations.Nicole Gribbin, Associate Director of Regulatory Affairs, Pharmaceuticals and Biologics, MCRA 03.19.24
-
FDA Looks to Industry for Collaboration and Harmonization
The FDA will no longer be a member of the Global Harmonization Working Party (GHWP).Rod Mell, Executive Head—Medical Devices, Regulatory Compliance Associates 02.05.24
-

Letter-to-File: Dealing with ‘Skeletons in the Closet’
In situations where LTF skeletons are hiding in the closet, honesty is always the best policy.Sarah Pleaugh, Associate Director of Regulatory Affairs, MCRA LLC 11.13.23
-
Materials

Navigating Nitinol’s Biocompatibility, Potential Toxicity, and the Evolving Regulatory Landscape
The material and market attributes make a clear case for nitinol’s broad applications in medical device manufacturing.Kim Ehman, Ph.D., Bindu Prabhakar, Ph.D., Dr. James Eucher, and Krississ Ohneswere, WuXi AppTec 09.12.23
-
The Silicon Valley Bank Collapse and Its Impact on Compliance
The collapse of SVB’s healthcare investment arm is likely to significantly impact the medical device industry, particularly for startups seeking financing.Linda Braddon, Ph.D., Founder and CEO, Secure BioMed Evaluations 05.23.23
-

Navigating the EU MDR UDI Requirements: The Benefits and Challenges
This is not an action that should be postponed until regulatory compliance is mandatory.Bayode Adisa, Manager, Regulatory Affairs – Europe, MCRA 03.10.23
-

FDA, EU MDR Updated Guidance Coming in 2023
The guidance is chock full of new measures for medtech manufacturers.Rod Mell, Executive Head – Medical Device Consulting, Regulatory Compliance Associates 02.17.23
-

Benefits and Implications of the Breakthrough Device Designation Program
As of June 30, CDRH and CBER have granted 693 Breakthrough Device designations.Palmer Smith, Associate, Spine Regulatory Affairs, MCRA LLC 11.15.22
-

The Case for Using Historical Control in Clinical Trial Design
Historical control refers to the use of data from past studies to estimate potential response to a placebo or standard care treatment among trial patients.Michael Coladonato and Lucas Tatem, MCRA 08.12.22
-

Breakthrough Device Designation’s Impact on Incremental Medicare Payment
There are impactful benefits that may allow for incremental reimbursement.John McDermott and Mickayla Roan, MCRA 05.26.22
-
Advice for a Successful Medical Device Global Registration Plan
One of the most powerful communication tools available to those responsible for placing medical devices on the market is a global registration plan.Kara Budor, M.S., Senior Manager, International Regulatory Affairs, MCRA LLC 02.14.22
-

FDA Regulatory Challenges for Antimicrobial Orthopedic Devices
Preventing and managing orthopedic-associated infections is one of the major challenges in orthopedic surgery.Mehdi Kazemzadeh-Narbat, Ph.D., Associate Director, Regulatory Affairs, MCRA LLC 11.17.21
-

The New U.K. Conformity Assessment: 10 Checklist Items
This new divergence between EU and U.K. requirements also means different rules apply to Great Britain (England, Scotland, and Wales) and Northern Ireland.Ed Ball, Senior Associate, RQM+ 09.14.21
-

Early Regulatory Strategy: Streamlining the Path to Market
Planning for a regulatory submission entails consideration of numerous requirements beyond simply the content and required elements of the application.Bryan Brosseau, Founder and Principal Consultant, Brosseau Consulting LLC 09.14.21
-

Five Lessons Learned Since MDR’s Implementation
The EU’s MDR took effect May 26, whereby all manufacturers must be in compliance if they intend to sell in the EU.Hannah Irwin and Michelle McDonough, MS, MCRA 08.17.21
-

When Is Digital Health Technology Regulated?
Whether you’re a startup or a well-established manufacturer, it can be challenging to determine if the software you’re developing needs FDA oversight.Nikki Batista, Director, Digital Health Regulatory Affairs, MCRA LLC 05.17.21
-

A Summary of Key Provisions Within CMS’ Final MCIT Rule
MCIT is available not only for new breakthrough devices but also for products that received this designation within the last two years.John D. McDermott Jr., Senior Director, Reimbursement Strategy, MCRA LLC 03.16.21
-
Biologics

An FDA Regulatory Perspective on Bone Grafts
There are various bone grafts with different composition, source, mechanical strength, and functional biological mechanisms.Mehdi Kazemzadeh-Narbat, Ph.D., Senior Associate, Regulatory Affairs, MCRA LLC 02.08.21
-
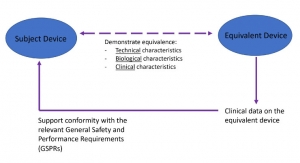
Medical Device Equivalence Requirements Strengthened under the EU MDR
This column focuses on the directive under EU 2017/745 on medical devices (MDR), highlighting some of the changes in claiming and demonstrating equivalence.Cindy Boyer, Senior Manager, International Regulatory Affairs, MCRA LLC 11.18.20
-

When to Submit a 510(k)
When are design and manufacturing changes considered significant enough to surpass the threshold of submitting a new 510(k)?Tom McDougal, MS, Associate, Regulatory Affairs, Musculoskeletal Clinical Regulatory Advisers LLC 09.15.20













