02.11.13
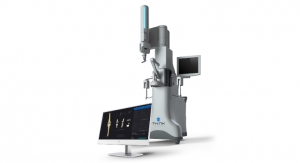
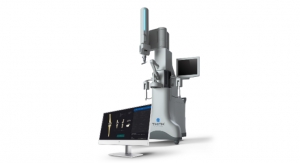
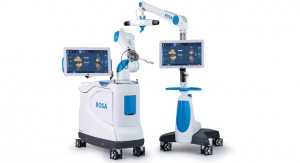
Warsaw, Ind.-based Zimmer Holdings Inc., a musculoskeletal health technology company, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market iAssist Knee, which the company calls a “personalized guidance system” for knee replacement procedures.
It is a surgical guidance system designed to provide improved intraoperative feedback and alignment validation to surgeons during joint replacement procedures. Current robotic and navigation systems use optical-tracking, requiring a clear line-of-sight into the surgical field, and rely upon complex additional equipment in the operating room, providing feedback on external computer screens.
The system, which Zimmer officials claim will keep the operating area less cluttered, is made up of small electronic disposable pod components that are used at the site of knee replacement surgery, providing alignment validation via an electronic display. The disposable pods are manipulated within the surgical field with positioning information provided by a series of internal accelerometers. iAssist Knee does not require the use of pins or additional incisions and does not rely on external systems or stimulus.
“iAssist Knee represents the next-step in intelligent instruments, offering significant benefits to patients, healthcare providers and health systems,” said Jeff McCaulley, president of Zimmer’s reconstructive business. “This innovative technology supports more streamlined and personalized knee replacement procedures through a simple, disposable intraoperative device. We are greatly excited by the potential of iAssist technology, which delivers on the promise of accurate implant positioning and alignment validation without the complexity, cost and time associated with current capital-intensive navigation and robotic systems.”
Zimmer makes orthopedic reconstructive, spinal and trauma devices, dental implants, and related surgical products.
Blue Belt Gets FDA Nod for Technology to Treat UKRs
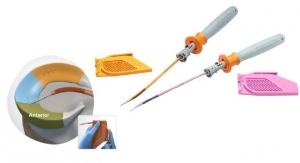
Blue Belt Technologies Inc. has received 510(k) clearance from the U.S. Food and Drug Administration to market its NavioPFS orthopedic surgical system. The system is cleared to perform unicondylar knee replacements (UKRs), often simply known as partial knee replacements.
A partial knee replacement is surgery to replace the osteoarthritic portion of the inside (medial) and/or outside (lateral) compartment of the knee with an implant prosthesis. The majority of partial knee replacement surgeries currently are performed using manual instruments that can, according to Blue Belt, be inconsistent and lead to high revision and retreatment rates.
The NavioPFS system incorporates smart instrumentation with robotic control to allow surgeons better precision. The system also delivers intra-operative navigation and 3-D visualization during the surgery based on a computed tomography-free registration process.
NavioPFS received CE marking for use in UKR surgeries from European regulators in February 2012. The company has completed clinical casework since then in Belgium and the United Kingdom where the system has produced positive early clinical results.
“We could not be any more excited to gain FDA clearance of our NavioPFS system,” said Eric B. Timko, president and CEO of Blue Belt Technologies. “The entire Blue Belt team, including our physician advisors, has remained committed to providing orthopedic surgeons and hospitals a more precise and consistent technique to perform UKR procedures that takes into consideration the current economic environment in our healthcare system. We are confident that NavioPFS accomplishes these goals, and provides excellent results for patients.”
Company officials said Blue Belt has begun selling NavioPFS in the United States for the UKR application, and the firm continues to develop other applications where the system can be used to perform precision bone-shaping procedures.
Based in Pittsburgh, Pa., Blue Belt Technologies develops smart surgical instruments with precision robotics for use in orthopedic procedures. Company officials hope to expand beyond the orthopedic market.
New Soft Tissue Repair Device from STR Cleared for Sale
New Haven, Conn.-based Soft Tissues Regeneration (STR), an early stage orthopedic device company that has developed a tissue engineering platform used to regenerate ligaments and tendons, has received U.S. Food and Drug Administration (FDA) clearance to market its STR graft, a biodegradable scaffold used for soft tissue augmentation and the repair of rotator cuffs.
The scaffold was developed by Cato T. Laurencin, M.D., Ph.D., orthopedic surgeon and founder of STR. The graft is a 3-D braided engineered matrix that Laurencin likens to a patch. During surgery, surgeons can drape this biodegradable patch over the tendon that sits on the shoulder bone, anchoring it with sutures to keep it in place while the tendon, bones and nearby tissues heal. Currently available devices are made of cadaver or animal tissue that can cause sutures to pull, while the STR Graft is thinner (about 1 millimeter) and reportedly stronger. STR claims these properties lessen pain, speed recovery time, and drastically reduce surgical failure rates.
Rotator cuff tears are a common cause of pain and disability among adults.
According to the American Academy of Orthopaedic Surgeons, in 2008, close to 2 million people in the United States saw their doctor because of a rotator cuff problem. Surgeries, however, are not always successful. According to Laurencin, depending on the size of the tear, the degree of muscle atrophy, the quality of the tendon, and the post-op rehabilitation protocol, repeat surgeries are necessary 20 percent to 70 percent of the time.
“There are several products available to augment rotator cuff repair, but they suffer from strength, suture pull-through and surgical deployment issues, all of which the STR graft addresses,”
Laurencin noted.
STR’s device has undergone several successful pre-clinical studies and extensive mechanical and other testing over the past two and a half years of development. The company expects to commercialize the graft by 2014.
FDA OKs Latest MIS Device from Precision Spine
The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the Mini-Max minimally invasive access system from Precision Spine Inc.
The Mini-Max fits into the firm’s growing range of minimally invasive spinal products. According to the company, the new system will enable spine surgeons to perform minimally invasive procedures using an “access/fixation” system that has been designed to achieve results that are the same as or better than those achieved using the current gold standard open-surgery procedures.
Mini-Max reportedly uses techniques that are familiar to surgeons, potentially shortening any learning curve and reducing operative time. Its hardware and corresponding procedural steps are configured to facilitate greater direct visualization of the spine and easier access to the contralateral side and levels above and below the target level.
In eliminating the need to remove the screw tulip head during assembly, the design team sought to reduce “fiddle factor” (time wasted with uncertainty on how to proceed) as well as the overall number of procedural steps. The system’s muscle-sparing technique, contrasted with percutaneous approaches that puncture muscle, is anticipated to speed recovery time and improve patient outcomes.
Precision’s design team focused on enabling more easily achieved procedure-to-procedure reproducibility, with the goal of enhancing cost-effectiveness for hospitals and payers.
“I believe that the incorporation of ‘minimally disruptive techniques’ familiar to spine surgeons, along with the ability to facilitate ‘maximum access’ through very small incision sites gives the new system significant advantages over the currently existing MIS spine systems now on the market,” said Donald Kucharzyk, M.D., director of the Minimally Invasive Spine Surgery Institute in Crown Point, Ind., and lead development surgeon for the new system.
Rich Dickerson, president of Precision Spine, said the device would be rolled out in two phases: “Phase 1, the base system, will enable pedicle screw-based tissue retraction and distraction for maximal access to the disc space. Phase 2, the additional system components, will enable parallel, bilateral distraction of vertebral bodies to facilitate even more effective placement of an advanced interbody device, which is now in the development phase.”
Precision Spine Inc. is headquartered in Parsippany, N.J., and has manufacturing facilities in Mississippi.
|
The iAssist guidance system. Photo courtesy of Zimmer Holdings Inc. |
The system, which Zimmer officials claim will keep the operating area less cluttered, is made up of small electronic disposable pod components that are used at the site of knee replacement surgery, providing alignment validation via an electronic display. The disposable pods are manipulated within the surgical field with positioning information provided by a series of internal accelerometers. iAssist Knee does not require the use of pins or additional incisions and does not rely on external systems or stimulus.
“iAssist Knee represents the next-step in intelligent instruments, offering significant benefits to patients, healthcare providers and health systems,” said Jeff McCaulley, president of Zimmer’s reconstructive business. “This innovative technology supports more streamlined and personalized knee replacement procedures through a simple, disposable intraoperative device. We are greatly excited by the potential of iAssist technology, which delivers on the promise of accurate implant positioning and alignment validation without the complexity, cost and time associated with current capital-intensive navigation and robotic systems.”
Zimmer makes orthopedic reconstructive, spinal and trauma devices, dental implants, and related surgical products.
Blue Belt Gets FDA Nod for Technology to Treat UKRs
|
Blue Belt’s NavioPFS orthopedic surgical system. Photo courtesy of Blue Belt. |
A partial knee replacement is surgery to replace the osteoarthritic portion of the inside (medial) and/or outside (lateral) compartment of the knee with an implant prosthesis. The majority of partial knee replacement surgeries currently are performed using manual instruments that can, according to Blue Belt, be inconsistent and lead to high revision and retreatment rates.
The NavioPFS system incorporates smart instrumentation with robotic control to allow surgeons better precision. The system also delivers intra-operative navigation and 3-D visualization during the surgery based on a computed tomography-free registration process.
NavioPFS received CE marking for use in UKR surgeries from European regulators in February 2012. The company has completed clinical casework since then in Belgium and the United Kingdom where the system has produced positive early clinical results.
“We could not be any more excited to gain FDA clearance of our NavioPFS system,” said Eric B. Timko, president and CEO of Blue Belt Technologies. “The entire Blue Belt team, including our physician advisors, has remained committed to providing orthopedic surgeons and hospitals a more precise and consistent technique to perform UKR procedures that takes into consideration the current economic environment in our healthcare system. We are confident that NavioPFS accomplishes these goals, and provides excellent results for patients.”
Company officials said Blue Belt has begun selling NavioPFS in the United States for the UKR application, and the firm continues to develop other applications where the system can be used to perform precision bone-shaping procedures.
Based in Pittsburgh, Pa., Blue Belt Technologies develops smart surgical instruments with precision robotics for use in orthopedic procedures. Company officials hope to expand beyond the orthopedic market.
New Soft Tissue Repair Device from STR Cleared for Sale
New Haven, Conn.-based Soft Tissues Regeneration (STR), an early stage orthopedic device company that has developed a tissue engineering platform used to regenerate ligaments and tendons, has received U.S. Food and Drug Administration (FDA) clearance to market its STR graft, a biodegradable scaffold used for soft tissue augmentation and the repair of rotator cuffs.
The scaffold was developed by Cato T. Laurencin, M.D., Ph.D., orthopedic surgeon and founder of STR. The graft is a 3-D braided engineered matrix that Laurencin likens to a patch. During surgery, surgeons can drape this biodegradable patch over the tendon that sits on the shoulder bone, anchoring it with sutures to keep it in place while the tendon, bones and nearby tissues heal. Currently available devices are made of cadaver or animal tissue that can cause sutures to pull, while the STR Graft is thinner (about 1 millimeter) and reportedly stronger. STR claims these properties lessen pain, speed recovery time, and drastically reduce surgical failure rates.
Rotator cuff tears are a common cause of pain and disability among adults.
According to the American Academy of Orthopaedic Surgeons, in 2008, close to 2 million people in the United States saw their doctor because of a rotator cuff problem. Surgeries, however, are not always successful. According to Laurencin, depending on the size of the tear, the degree of muscle atrophy, the quality of the tendon, and the post-op rehabilitation protocol, repeat surgeries are necessary 20 percent to 70 percent of the time.
“There are several products available to augment rotator cuff repair, but they suffer from strength, suture pull-through and surgical deployment issues, all of which the STR graft addresses,”
Laurencin noted.
STR’s device has undergone several successful pre-clinical studies and extensive mechanical and other testing over the past two and a half years of development. The company expects to commercialize the graft by 2014.
FDA OKs Latest MIS Device from Precision Spine
The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the Mini-Max minimally invasive access system from Precision Spine Inc.
The Mini-Max fits into the firm’s growing range of minimally invasive spinal products. According to the company, the new system will enable spine surgeons to perform minimally invasive procedures using an “access/fixation” system that has been designed to achieve results that are the same as or better than those achieved using the current gold standard open-surgery procedures.
Mini-Max reportedly uses techniques that are familiar to surgeons, potentially shortening any learning curve and reducing operative time. Its hardware and corresponding procedural steps are configured to facilitate greater direct visualization of the spine and easier access to the contralateral side and levels above and below the target level.
In eliminating the need to remove the screw tulip head during assembly, the design team sought to reduce “fiddle factor” (time wasted with uncertainty on how to proceed) as well as the overall number of procedural steps. The system’s muscle-sparing technique, contrasted with percutaneous approaches that puncture muscle, is anticipated to speed recovery time and improve patient outcomes.
Precision’s design team focused on enabling more easily achieved procedure-to-procedure reproducibility, with the goal of enhancing cost-effectiveness for hospitals and payers.
“I believe that the incorporation of ‘minimally disruptive techniques’ familiar to spine surgeons, along with the ability to facilitate ‘maximum access’ through very small incision sites gives the new system significant advantages over the currently existing MIS spine systems now on the market,” said Donald Kucharzyk, M.D., director of the Minimally Invasive Spine Surgery Institute in Crown Point, Ind., and lead development surgeon for the new system.
Rich Dickerson, president of Precision Spine, said the device would be rolled out in two phases: “Phase 1, the base system, will enable pedicle screw-based tissue retraction and distraction for maximal access to the disc space. Phase 2, the additional system components, will enable parallel, bilateral distraction of vertebral bodies to facilitate even more effective placement of an advanced interbody device, which is now in the development phase.”
Precision Spine Inc. is headquartered in Parsippany, N.J., and has manufacturing facilities in Mississippi.