Michael Barbella, Managing Editor11.30.18
Camil Moldoveanu was fully prepared for the challenge. Or so he thought.
The four-time Brazilian jiu-jitsu world champion knew he would need significant rehabilitation for his knee injury. He also knew the recovery process would be difficult, and oftentimes physically taxing.
But he didn’t know—and thus, wasn’t prepared for—the confusion that eventually sidelined his recovery.
“Nobody could explain to me why I needed to do eight weeks [of rehabilitation] when I felt my knee was strong enough,” Moldoveanu said recently from the exhibit hall floor of Medica 2018, the world’s largest medical trade fair. “When I first went home, the doctor told me to flex my knee regularly because if I didn’t, the calcium could build up and I would need surgery. I asked to what degree I should flex my knee and he said ‘not too great but enough so there’s some motion going on. I said, ‘what does that mean?’”
Lacking treatment specifics and a dedicated team to monitor his recovery, Moldoveanu suffered the same fate as other rehab skimpers: He injured his right meniscus again and had to undergo surgery. That recovery process, however, took six months.
“When you self-report at the doctor’s office, you’re asked, ‘Hey, have you done all your exercises?’ Moldoveanu explained. “And you say, ‘Yes, of course.’”
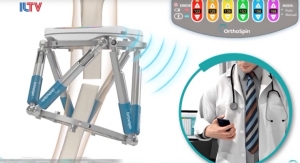
Moldoveanu’s disjointed and directionless rehabilitation experience led him to start a company that could ease the rehabilitation process and clear up any confusion about treatment. His firm, re.flex, is developing a motion-tracking wearable that guides patients during the last stages of physiotherapy rehabilitation, helping them perform home exercises effectively. The wearable has been tested by patients who have undergone TKR/THR, meniscus reconstructions, and/or surgery to repair ligament tears.
The re.flex wearable tracks up to 20 parameters of hip and knee movement such as flexion, extension, shin rotation, speed, acceleration, abduction, or adduction. It can track movement when the patient is lying down, and half the knee exercises that require posture, said Moldoveanu, who is also the company’s CEO.
The re.flex device attaches to each side of the injured joint; during exercises, the wearables collect data and provide real-time feedback to clinicians, who monitor and alert patients to any improperly performed movements. The device also allows doctors to track patients’ progress online and change routines as necessary.
re.flex currently is working with several clinics and hospitals in Germany, Romania, and the United Kingdom, and hopes to expand its base on the promise of cost reduction as well as improved patient recovery.
“The physiotherapists who have been using re.flex are amazed by how well the device is working and how easy it is for patients to use it,” Moldoveanu told ZDNet.
Orthopedic-focused technology like re.flex was a rarity at Medica this year, as such innovation was easily overshadowed by the throngs of laboratory/diagnostic solutions, patient monitoring devices, scanning equipment, hospital furniture, and physical therapy machines on display during the four-day event in Düsseldorf, Germany. Even industry behemoths like Stryker Corp.—which usually spares no expense with its bombastic exhibit hall booths—was a shadow of its former self at the show, displaying its wares in a significantly smaller confine than other firms (and countries).
Nevertheless, there was an engaging array of innovation on hand that provided a glimpse into the future of the industry. Limfa Technologies Ltd., for example, was plugging its cell repair technology for tissue regeneration and healing. The firm’s LIMFA therapy system uses 29 patented biomagnetic signals to simulate the self-repair mechanism in human cells, accelerating their repair with an 80 percent proven efficacy rate.
“It’s working at the cell level, it doesn’t share energy with the body,” managing director Tommaso Faiella told Orthopedic Design & Technology. “We have had a high success rate with soft tissue, muscles, and nerves. Each kind of tissue responds to different speeds. It works the same way as a drug—we have the same aim, but there is no side effects with our therapy.”
LIMFA therapy is CE-marked and currently sold only in Italy (where the firm is based), but will soon be distributed in other European regions. Faiella said the company plans to market the product for cartilage repair next year.
In a nearby hall, meanwhile, Fraunhofer researchers showcased a virtual reality system for temple bone drilling, individualized osteoporosis therapy, and implant antibacterial coating technology using silver ions.
The virtual reality system, not yet approved for clinical use, featured a 3D monitor suspended over a large-sized mirror to simulate microscopic drilling into the temple bone (behind the ear). The researchers’ technology provides users with clearly marked boundaries for drilling as well as a realistic feeling of drilling into bone.
“You not only see the bone, you feel the bone too,” noted Thomas Eixelberger, M.Sc., of Fraunhofer’s Biomedical Research Group. “Normally, this surgery is done under a microscope so the surgeon can see structures like the facial nerve. We used a mirror because when you’re working under a microscope, you don’t see your hands, and you can’t see your hands under the mirror. It’s just like it is in the real world.”
The real world also inspired Fraunhofer’s OsteoSys project, which is studying the link between cardiovascular disease, inflammation, and bone metabolism. Researchers hope to use their findings to provide personalized therapy to patients that inhibit bone depletion and minimize drug-related side effects.
The four-time Brazilian jiu-jitsu world champion knew he would need significant rehabilitation for his knee injury. He also knew the recovery process would be difficult, and oftentimes physically taxing.
But he didn’t know—and thus, wasn’t prepared for—the confusion that eventually sidelined his recovery.
“Nobody could explain to me why I needed to do eight weeks [of rehabilitation] when I felt my knee was strong enough,” Moldoveanu said recently from the exhibit hall floor of Medica 2018, the world’s largest medical trade fair. “When I first went home, the doctor told me to flex my knee regularly because if I didn’t, the calcium could build up and I would need surgery. I asked to what degree I should flex my knee and he said ‘not too great but enough so there’s some motion going on. I said, ‘what does that mean?’”
Lacking treatment specifics and a dedicated team to monitor his recovery, Moldoveanu suffered the same fate as other rehab skimpers: He injured his right meniscus again and had to undergo surgery. That recovery process, however, took six months.
“When you self-report at the doctor’s office, you’re asked, ‘Hey, have you done all your exercises?’ Moldoveanu explained. “And you say, ‘Yes, of course.’”
Moldoveanu’s disjointed and directionless rehabilitation experience led him to start a company that could ease the rehabilitation process and clear up any confusion about treatment. His firm, re.flex, is developing a motion-tracking wearable that guides patients during the last stages of physiotherapy rehabilitation, helping them perform home exercises effectively. The wearable has been tested by patients who have undergone TKR/THR, meniscus reconstructions, and/or surgery to repair ligament tears.
The re.flex wearable tracks up to 20 parameters of hip and knee movement such as flexion, extension, shin rotation, speed, acceleration, abduction, or adduction. It can track movement when the patient is lying down, and half the knee exercises that require posture, said Moldoveanu, who is also the company’s CEO.
The re.flex device attaches to each side of the injured joint; during exercises, the wearables collect data and provide real-time feedback to clinicians, who monitor and alert patients to any improperly performed movements. The device also allows doctors to track patients’ progress online and change routines as necessary.
re.flex currently is working with several clinics and hospitals in Germany, Romania, and the United Kingdom, and hopes to expand its base on the promise of cost reduction as well as improved patient recovery.
“The physiotherapists who have been using re.flex are amazed by how well the device is working and how easy it is for patients to use it,” Moldoveanu told ZDNet.
Orthopedic-focused technology like re.flex was a rarity at Medica this year, as such innovation was easily overshadowed by the throngs of laboratory/diagnostic solutions, patient monitoring devices, scanning equipment, hospital furniture, and physical therapy machines on display during the four-day event in Düsseldorf, Germany. Even industry behemoths like Stryker Corp.—which usually spares no expense with its bombastic exhibit hall booths—was a shadow of its former self at the show, displaying its wares in a significantly smaller confine than other firms (and countries).
Nevertheless, there was an engaging array of innovation on hand that provided a glimpse into the future of the industry. Limfa Technologies Ltd., for example, was plugging its cell repair technology for tissue regeneration and healing. The firm’s LIMFA therapy system uses 29 patented biomagnetic signals to simulate the self-repair mechanism in human cells, accelerating their repair with an 80 percent proven efficacy rate.
“It’s working at the cell level, it doesn’t share energy with the body,” managing director Tommaso Faiella told Orthopedic Design & Technology. “We have had a high success rate with soft tissue, muscles, and nerves. Each kind of tissue responds to different speeds. It works the same way as a drug—we have the same aim, but there is no side effects with our therapy.”
LIMFA therapy is CE-marked and currently sold only in Italy (where the firm is based), but will soon be distributed in other European regions. Faiella said the company plans to market the product for cartilage repair next year.
In a nearby hall, meanwhile, Fraunhofer researchers showcased a virtual reality system for temple bone drilling, individualized osteoporosis therapy, and implant antibacterial coating technology using silver ions.
The virtual reality system, not yet approved for clinical use, featured a 3D monitor suspended over a large-sized mirror to simulate microscopic drilling into the temple bone (behind the ear). The researchers’ technology provides users with clearly marked boundaries for drilling as well as a realistic feeling of drilling into bone.
“You not only see the bone, you feel the bone too,” noted Thomas Eixelberger, M.Sc., of Fraunhofer’s Biomedical Research Group. “Normally, this surgery is done under a microscope so the surgeon can see structures like the facial nerve. We used a mirror because when you’re working under a microscope, you don’t see your hands, and you can’t see your hands under the mirror. It’s just like it is in the real world.”
The real world also inspired Fraunhofer’s OsteoSys project, which is studying the link between cardiovascular disease, inflammation, and bone metabolism. Researchers hope to use their findings to provide personalized therapy to patients that inhibit bone depletion and minimize drug-related side effects.