Maria Shepherd, President and Founder, Medi-Vantage09.15.20
Over the past 10 years, medtech manufacturers from startups to giants like Zimmer, Stryker, and Smith+Nephew have grown their levels of outsourcing to contract R&D and manufacturing partners. The objective of this market shift is to gain operational efficiencies, cost savings, and access to new capabilities that provide a competitive advantage.
Orthopedic device companies have pivoted to concentrate on their internal core competencies. Under these conditions, outsourcing makes sense in terms of cost and speed to market if orthopedic device manufacturers select contract R&D and manufacturing partners that can expand their in-house innovation capabilities, offer scale and quality improvement, and push the fixed costs of internal R&D to the other side of the balance sheet.
The drivers of this market are continued industry consolidation (driven by hospital consolidation with more expected in the future), painful cuts in reimbursement, and the driving need to rapidly innovate. Competition is increasing from every direction such as new and emerging adjacent market players like Apple and Google, digital technologies, crossover from pharmaceutical companies in the wearables space, and unknown and possibly underestimated sources of risk such as Haven Healthcare—led by Atul Gawande, M.D., MPH, and chairman of the board, which is focused on the U.S.-based employees and families from Amazon, Berkshire Hathaway, and JPMorgan Chase.
Why This Is Important
Contract medical device R&D has emerged as an industry of its own, with expertise at all stages of the medtech lifecycle. Sheer scale is part of the reason. FDA’s CDRH reports there are approximately 175,000 categories of medical devices available in the U.S.1 Some reports put global annual medtech sales at $425 billion in 2018 and its CAGR was forecasted (pre-COVID) to grow at approximately 5.4 percent for the near future, placing the market in 2027 at approximately $680 billion.2 Few medical device developers have the basic but broad technical expertise and resources to develop products in-house. There is a high cost to acquire that required infrastructure and expertise.
Orthopedic device companies have limited finances (and all have limited budgets, especially now, in the COVID era) to maintain and manage complicated R&D programs. In addition, regulations are changing. Human factors usability testing has been mandatory since 2016 and the EU’s revised MDR will require medical devices to undergo exacting quality programs, which further escalates the need for expertise and dedicated resources. COVID-19 has delayed the enforcement of the EU’s MDR until May 2021, which will be here before we know it, so this is a “can” medical device companies cannot continue to kick down the road. Medtech manufacturers also expect outsourcing to yield cost savings and faster time to market. These are all drivers of the outsourcing of R&D, manufacturing, clinical research, and regulatory affairs.
This growth will be substantial. For example, the global medtech contract manufacturing market is expected to grow to almost $100 billion by 2027 (Table 1), a CAGR of approximately 7 percent.3
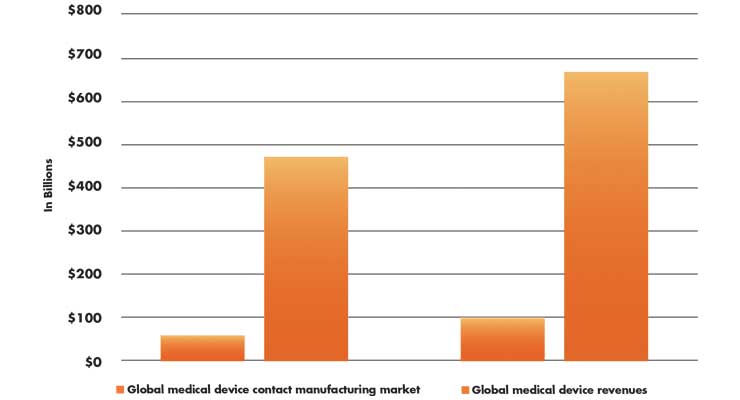
Table 1: June 2019 to June 2020 mobile operating system market share worldwide3
Specialization in the Medtech Outsourced Services Market
According to a recent report by MassMEDIC, contract R&D services are being specialized in recognition of the need for specific services by medical specialty.4 The areas of highest growth are drug delivery, IVD consumables, and robotic-assisted surgical systems (Table 2). These are the areas where market demand is proven and has shown sustainability. The two largest markets—cardiovascular and orthopedic devices—may require high-performance materials, manufacturing, or end-use market demand, such as digital health or drug delivery.
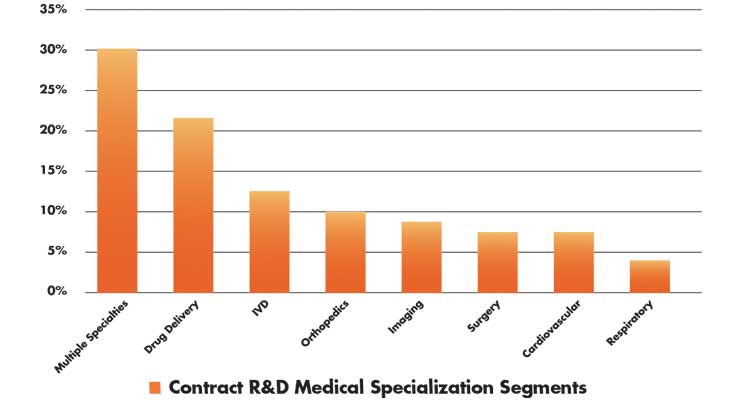
Table 2: 2025 expected market segments for contract R&D specialized focus4
Critical Success Factors
While the orthopedic device contract R&D and manufacturing market is highly fragmented, there are competitive trends all must recognize to be successful. Expect and plan for the following:
As noted in HS&M magazine, 2019 was a year of exceptional organic sales growth and a five-year nadir for acquisitions with some notable recent exceptions for orthopedic manufacturers (Table 3). Medtronic bought Israeli robotic spine surgery company Mazor Robotics for $1.7 billion, and Stryker acquired spine company K2M for $1.4 billion, and then followed up with the purchase of six other companies. Wright Medical bought Cartiva, a manufacturer of synthetic cartilage implants for $435 million and Smith+Nephew acquired the NovoStitch Pro to address complex meniscal tear repair through its $105 million acquisition Ceterix Orthopaedics.
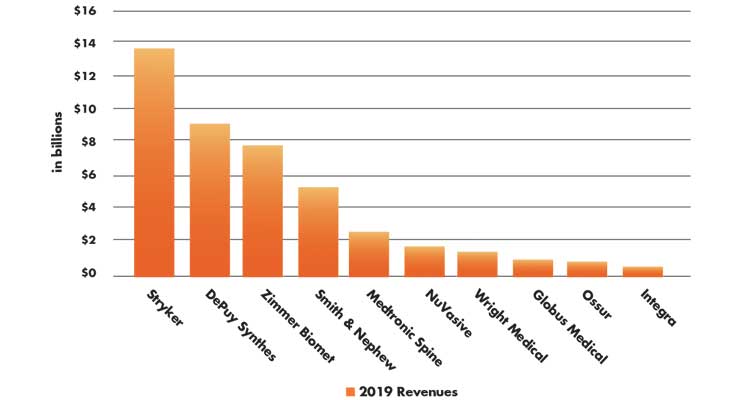
Table 3: 2019 orthopedic company global revenues5
The Medi-Vantage Perspective
The medtech industry saw an enormous amount of growth in 2019, and it is anyone’s guess where we will end on Dec. 31, 2020. Only nine of the top 50 medical device companies listed in 2019 by HS&M saw revenue decreases.6 Investment in medical device R&D expanded two times during the 1990s but since then, R&D investment has dropped to 4.7 percent by 2017 from an average of 15.5 percent between 2000 and 2007. If the medtech trend of under-investing in R&D continues, what happens to the dominant position of the U.S. in medical device innovation? Innovate, know your customer, and take calculated risks.
References
Maria Shepherd has more than 20 years of leadership experience in medical device/life-science marketing in small startups and top-tier companies. After her industry career, including her role as vice president of marketing for Oridion Medical where she boosted the company valuation prior to its acquisition by Medtronic, director of marketing for Philips Medical, and senior management roles at Boston Scientific Corp., she founded Medi-Vantage. Medi-Vantage provides marketing and business strategy and innovation research for the medical device industry. The firm quantitatively and qualitatively sizes and segments opportunities, evaluates new technologies, provides marketing services, and assesses prospective acquisitions. Shepherd has taught marketing and product development courses, speaks regularly at medtech conferences, and can be reached at mshepherd@medi-vantage. Visit her website at www.medi-vantage.com.
Orthopedic device companies have pivoted to concentrate on their internal core competencies. Under these conditions, outsourcing makes sense in terms of cost and speed to market if orthopedic device manufacturers select contract R&D and manufacturing partners that can expand their in-house innovation capabilities, offer scale and quality improvement, and push the fixed costs of internal R&D to the other side of the balance sheet.
The drivers of this market are continued industry consolidation (driven by hospital consolidation with more expected in the future), painful cuts in reimbursement, and the driving need to rapidly innovate. Competition is increasing from every direction such as new and emerging adjacent market players like Apple and Google, digital technologies, crossover from pharmaceutical companies in the wearables space, and unknown and possibly underestimated sources of risk such as Haven Healthcare—led by Atul Gawande, M.D., MPH, and chairman of the board, which is focused on the U.S.-based employees and families from Amazon, Berkshire Hathaway, and JPMorgan Chase.
Why This Is Important
Contract medical device R&D has emerged as an industry of its own, with expertise at all stages of the medtech lifecycle. Sheer scale is part of the reason. FDA’s CDRH reports there are approximately 175,000 categories of medical devices available in the U.S.1 Some reports put global annual medtech sales at $425 billion in 2018 and its CAGR was forecasted (pre-COVID) to grow at approximately 5.4 percent for the near future, placing the market in 2027 at approximately $680 billion.2 Few medical device developers have the basic but broad technical expertise and resources to develop products in-house. There is a high cost to acquire that required infrastructure and expertise.
Orthopedic device companies have limited finances (and all have limited budgets, especially now, in the COVID era) to maintain and manage complicated R&D programs. In addition, regulations are changing. Human factors usability testing has been mandatory since 2016 and the EU’s revised MDR will require medical devices to undergo exacting quality programs, which further escalates the need for expertise and dedicated resources. COVID-19 has delayed the enforcement of the EU’s MDR until May 2021, which will be here before we know it, so this is a “can” medical device companies cannot continue to kick down the road. Medtech manufacturers also expect outsourcing to yield cost savings and faster time to market. These are all drivers of the outsourcing of R&D, manufacturing, clinical research, and regulatory affairs.
This growth will be substantial. For example, the global medtech contract manufacturing market is expected to grow to almost $100 billion by 2027 (Table 1), a CAGR of approximately 7 percent.3

Table 1: June 2019 to June 2020 mobile operating system market share worldwide3
Specialization in the Medtech Outsourced Services Market
According to a recent report by MassMEDIC, contract R&D services are being specialized in recognition of the need for specific services by medical specialty.4 The areas of highest growth are drug delivery, IVD consumables, and robotic-assisted surgical systems (Table 2). These are the areas where market demand is proven and has shown sustainability. The two largest markets—cardiovascular and orthopedic devices—may require high-performance materials, manufacturing, or end-use market demand, such as digital health or drug delivery.

Table 2: 2025 expected market segments for contract R&D specialized focus4
Critical Success Factors
While the orthopedic device contract R&D and manufacturing market is highly fragmented, there are competitive trends all must recognize to be successful. Expect and plan for the following:
- Technical differentiation is a critical success factor that allows medical device contract R&D and manufacturing providers to maintain prices or charge a slight price premium.
- Vertically integrated competitors recognize and are aligning their core strengths and footprint with the medical specialty they serve.
- More consolidation in the medical device contract R&D and manufacturing industry is coming, following the trends set by hospitals and medtech manufacturers.
- Target early in product development, while emphasizing strengths upstream, with a high focus on quality to be able to serve orthopedic device manufacturers throughout the lifecycle of the device.
- Establish long-term partnerships that can serve as a highly credible proof source to expand business at large orthopedic device manufacturers.
- Maintain margins through outsourcing less-competitive R&D activities.
As noted in HS&M magazine, 2019 was a year of exceptional organic sales growth and a five-year nadir for acquisitions with some notable recent exceptions for orthopedic manufacturers (Table 3). Medtronic bought Israeli robotic spine surgery company Mazor Robotics for $1.7 billion, and Stryker acquired spine company K2M for $1.4 billion, and then followed up with the purchase of six other companies. Wright Medical bought Cartiva, a manufacturer of synthetic cartilage implants for $435 million and Smith+Nephew acquired the NovoStitch Pro to address complex meniscal tear repair through its $105 million acquisition Ceterix Orthopaedics.

Table 3: 2019 orthopedic company global revenues5
The Medi-Vantage Perspective
The medtech industry saw an enormous amount of growth in 2019, and it is anyone’s guess where we will end on Dec. 31, 2020. Only nine of the top 50 medical device companies listed in 2019 by HS&M saw revenue decreases.6 Investment in medical device R&D expanded two times during the 1990s but since then, R&D investment has dropped to 4.7 percent by 2017 from an average of 15.5 percent between 2000 and 2007. If the medtech trend of under-investing in R&D continues, what happens to the dominant position of the U.S. in medical device innovation? Innovate, know your customer, and take calculated risks.
References
Maria Shepherd has more than 20 years of leadership experience in medical device/life-science marketing in small startups and top-tier companies. After her industry career, including her role as vice president of marketing for Oridion Medical where she boosted the company valuation prior to its acquisition by Medtronic, director of marketing for Philips Medical, and senior management roles at Boston Scientific Corp., she founded Medi-Vantage. Medi-Vantage provides marketing and business strategy and innovation research for the medical device industry. The firm quantitatively and qualitatively sizes and segments opportunities, evaluates new technologies, provides marketing services, and assesses prospective acquisitions. Shepherd has taught marketing and product development courses, speaks regularly at medtech conferences, and can be reached at mshepherd@medi-vantage. Visit her website at www.medi-vantage.com.