Sam Brusco, Associate Editor11.17.21
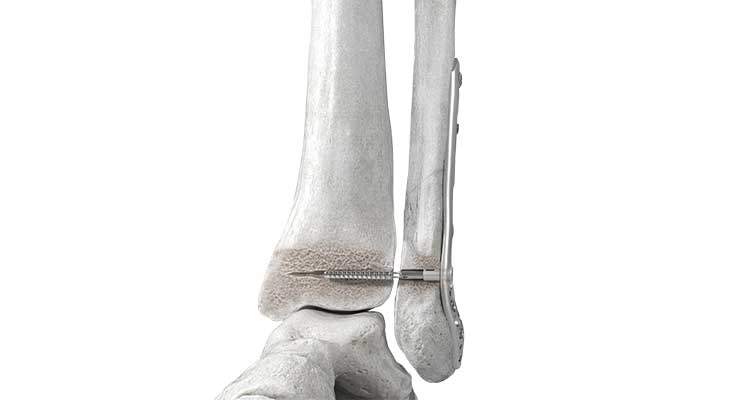
At this past October’s American Society for Surgery of the Hand annual meeting, Bedford, Mass.-based Anika Therapeutics released its WristMotion Total Wrist Arthroplasty (TWA) system, which it acquired from joint surface and preservation solutions maker Arthrosurface in 2020. Used to alleviate pain and restore arthritic wrist joint function, its design maximizes carpal stability and enables rotational freedom—including the “dart thrower’s” motion.
“Historically, patients suffering with advanced wrist pain caused by arthritis or trauma were treated with older versions of total wrist arthroplasty systems or with wrist fusions,” Arnold-Peter C. Weiss, M.D., chief - Hand, Upper Extremity & Microvascular Surgery, vice chairman and professor of Orthopaedics, Warren Alpert Medical School, Brown University, told the press. “Existing total wrist arthroplasty implants on the market today may enable some wrist motion but are at higher risk of carpal loosening. Alternatively, a wrist fusion may severely limit a patient’s ability to move and use their wrist. Neither option restores wrist function, and that’s the core reason the WristMotion TWA System is a significant step forward for surgeons and patients.”
WristMotion TWA leverages over 12 years of data focused on stability and improving wrist implants with a proprietary taper post-fixation for long-term stability, and anatomic design for motion preservation. The modular joint preservation system replaces the radial and carpal sides of the wrist joint. A dual curvature carpal design feature combined with the dorsal flange allows for greater range of extension due to increased implant surface area, according to the company. Early clinical feedback on instrumentation, surgical technique, and post-op range of motion has been positive. “The WristMotion TWA System’s proprietary central threaded taper post helps ensure secure carpal fixation,” William B. Geissler, M.D., Alan E. Freeland Chair of Hand Surgery, professor and chief, Division of Hand and Upper Extremity, University of Mississippi, told the press. “This secure carpal fixation, coupled with the ability to adjust the height and tension of the wrist joint on the radial side rather than carpal, strengthens the implant’s stability and also preserves the natural center of rotation.” Geissler was the first surgeon in Mississippi to implant the WristMotion TWA System. “It is truly the next generation of total wrist arthroplasty.”
Moving up the ladder a bit, ODT Top 10 denizen DJO launched the MedShape DynaNail Hybrid Fusion System this past September. The system leverages active, adaptive healing technology to maintain dynamic compression at the fusion site with an anatomically friendly design that simplifies insertion. Its combined screw/nail design provides a balance of ease and reliability. A threaded, screw-like tip complements bone anatomy and the distal transverse calcaneal screw offers stability and prevents device migration. Maintained active compression is achieved via proprietary Internal Superelastic NiTiNOL Element, which responds to up to 5 mm of bone settling or resorption at the site of healing depending on the length of the implant.
“The DynaNail Hybrid provides the maintained active compression necessary for successful fusion in a package designed specifically to complement subtalar anatomy,” Dr. Samuel Adams of Duke University Medical Center told the press. “Not only can I implant with ease and efficiency, but the active, adaptive healing approach instills confidence that my patients will experience the best possible outcomes.”
According to Technavio, the extremities market is poised to grow to $3.24 billion, progressing at a CAGR of over 8 percent between 2021 and 2025. The extremity orthopedics market differs from the hip and knee sectors, where major players comprise a majority of the market. The market is fragmented, with several players occupying it, and is segmented by upper (shoulder, elbow, hand/wrist) and lower (foot/ankle) extremities. The upper extremities segment accrued maximum revenue, according to Technavio, due to the increasing incidence of work-related injuries and road accidents.
The North American market, according to the market research firm, will be responsible for 44 percent of growth this year. This was mainly driven by presence of favorable reimbursement programs such as the Comprehensive Care for Joint Replacement (CJR) Model, the Surgical Hip and Fracture Treatment (SHFFT) Model, and the Merit-based Incentive Payment System (MIPS) Model in the region.
Adoption of 3D printing and incorporation of robotics are increasing efficiency and reducing time required for surgeries, and customizing implants according to the patient’s anatomy. The technological advances are increasing extremities products adoption among end-users.
Although the market is quite fractured, this article will examine how two global orthopedic device manufacturers and one manufacturing partner are addressing the extremity orthopedics industry.
DePuy Synthes
Global orthopedic device manufacturer DePuy Synthes touts a wide variety of extremity products in its portfolio. Its upper extremities arsenal contains technologies to assist with shoulder, wrist, elbow, and clavicle fractures. The foot and ankle franchise targets foot, ankle, and calcaneal fractures, as well as hammertoe, hallux valgus, first MTP (metatarsophalangeal) fusion, midfoot arthrodesis, flatfoot reconstruction, and deformity correction. The company also harbors technologies to address shoulder repair and replacement.
“Extremities is a complex space spanning both upper and lower extremities that has tremendous potential to address multiple unmet needs for patients, surgeons, and providers,” said Oray Boston, worldwide president, DePuy Synthes TECA (Trauma, Extremities, Craniomaxillofacial and Animal Health). “We focus on innovation with the goal to improve clinical outcomes for patients and surgeons, while supporting providers’ needs in this rapidly evolving healthcare environment. We provide solutions, both with implants and surgical process perspectives, that have the potential to improve bone healing, increase surgical precision, increase intra-operative flexibility, and reduce post-operative complications.”
Elective surgery restrictions negatively impacted ambulatory surgical center (ASC) finances in early 2020, but the industry largely recovered rapidly and was poised to accept a greater share of procedures this year. Driven to minimize healthcare costs, insurers were already attempting to steer procedures to ASCs instead of costlier hospitals prior to the COVID-19 pandemic. Infection concerns due to the pandemic are making ASCs an even more popular choice for patients, as well. Becker’s ASC Review predicted more partnerships formed between existing surgery centers and hospitals and more joint-venture centers between independent practices and hospitals.
“As more and more extremities surgeries take place outside of large hospitals, we are putting specialized teams in place that are working to better understand the needs of customers to support sites of care such as ASCs,” said Boston. “Having deep understanding of their needs allows us to provide custom tailored solutions to meet their requirements.”
The company touts an extensive portfolio of extremity orthopedic products.
“Extremities is an area of high strategic importance where we have made significant investments, both in portfolio innovation and commercial infrastructure, said Boston. “Just in the past two years we have launched new headless compression screws, a radial head replacement system, DePuy Synthes Hammertoe Continuous Compression Implant, FIBULINK Syndesmosis Repair System, and the Variable Angle LCP Periprosthetic Proximal Femur Plating System.”
The company’s headless compression screws are self-drilling and self-tapping for surgeon-controlled compression and simplified technique. The smallest of the screws (1.5 mm) can be used for DIP arthrodesis, metacarpal base or head fracture, and Weil osteotomy (distal oblique osteotomy of the metatarsal head). The next sizes up (2.4 mm and 3 mm) are used for PIP arthrodesis, condylar fracture radial styloid fracture, and Chevron osteotomy.
“The headless compression screw system is the most comprehensive portfolio in its class on the market to address a wide range of surgical trauma and elective procedures,” said Boston. “This system offers titanium alloy, self-drilling/self-tapping screws that feature reverse cutting flutes, differential thread pitch and offer uncompromised precision through an innovative cutting edge for improved cutting efficiency.”
DePuy Synthes’ extramedullary implant for hammertoe fixation was designed with Nitinol, according to company, for continuous compression and superior rotational stability with greater distraction resistance. Designed for small bone reconstruction and fusion of the phalanges in toes, the extramedullary configuration provides bone-stock preservation and easy removal as well. The technology was added to the company’s portfolio from its acquisition of Biomedical Enterprises Inc. in 2016.
“The DePuy Synthes Hammertoe Continuous Compression Implant is the first extramedullary continuous compression implant on the market designed to provide greater stability, preservation of bone stock, and easier removal than intramedullary devices for the treatment of Hammertoe, which occurs as a result of a muscle imbalance that puts pressure on the toe tendons and joints,” said Boston. “Muscles work in pairs to straighten and bend the toes. If the toe is bent in one position long enough, the muscles and joints tighten and cannot stretch out.”
The firm’s radial head replacement system was launched last October for patients with destabilizing radial head fractures that can’t be repaired. It can also be used for elective management of elbow arthritis. The smooth, stemmed implants were designed to allow for unrestricted motion, allowing the stem to self-center inside the radial canal.
“The Radial Head Replacement System is used to treat radial head fractures and is the first with color-coded radiolucent trials that allow in-situ height determination and visualization of proximal ulna and joint,” said Boston. “It features a smooth stem design, which may enhance the implant’s ability to self-align and dial-in when the elbow goes through a range of motion and may articulate with the capitellum throughout the entire arc of motion.”
October 2020 saw the release of the company’s FIBULINK Syndesmosis Repair System to enable precise tension control and physiologic motion for patients with traumatic ankle injuries. The technology was acquired earlier in 2020 from Akros Medical. According to DePuy Synthes, its high-strength suture bridge eliminates complication risks associated with broken synesmotic screws. Syndesmotic fixation can be performed without a medial incision or hardware, minimizing medial side complications like neurovascular structure damage or soft tissue entrapment. The implants come in stainless steel or titanium and are compatible with the firm’s distal fibula plates and any plate hole that accepts a 4 mm non-locking cortex screw.
“The FIBULINK Syndesmosis Repair System is the only flexible syndesmotic repair system that has the ability to fine tune and readjust tension intraoperatively,” said Boston. “It eliminates medial soft tissue disruption and helps improve procedural efficiency by delivering fixation through a single lateral incision (compared to suture button constructs).”
The company’s 2.7 mm VA LCP (variable angle locking compression plate) Clavicle Plate System hit the market this past June, providing tools to treat lateral, shaft, and medial fractures for small, medium, and large clavicles. To develop the technology the company observed over 600 clavicle CT scans from a broad-based population of patients while analyzing 15 separate bone parameters to identify correlation between patient height and clavicle shape.
“The VA LCP Clavicle Plate System offers plate shapes that reflect the correlation between patient stature and clavicle size to match the clavicle’s bow and contour and accommodate a broad range of anatomic variability for the treatment of collarbone fractures. This new system offers thinner plates, a more accurate plate to bone fit, and reduced prominence.”
Smith+Nephew
Global medical device manufacturer Smith+Nephew also touts an impressive array of extremity orthopedic surgery products. These include a wide variety of small joint surgical instruments, suture anchors, hand and wrist joint fusion, repair, and replacement technologies, shoulder repair and replacement implants, and foot/ankle fixation, fusion, and joint replacement products.
“We offer advanced materials designed for durability and longevity in joint replacement surgery,” said Leigh Lovato, senior director of global marketing, Trauma & Extremities, for Smith+Nephew. “Orthopedic joint replacements historically are made out of traditional metallic alloys and polymers. Over time, metals can wear polymers, which may result in a shorter device lifespan.”
To perform ankle replacement surgery, the tibia and talus surface making up the tibiotalar joint is removed and metal components are inserted into the bone. A plastic insert is attached to the tibial metal piece. The metal piece on the talus will rub against the plastic piece attached to the tibial implant, removing ankle arthritis pain and preserving joint motion.
“Total ankle replacement is a historically complex procedure with a steep learning curve,” said Lovato. “Compared to large joint replacements such as hips and knees, procedural volume of ankle replacement procedures per year is relatively low. These factors drive the need for implant and instrument design simplicity to enable a more reproducible and predictable procedure with the goal of improving patient outcomes. Our goal is to satisfy this need through new product innovation and enabling peer-to-peer educational opportunities.”
The company rolled out the CADENCE Total Ankle Flat Cut Talar Dome System this past August. This total ankle replacement addition to Smith+Nephew’s portfolio was the result of acquiring Integra LifeScience’s extremity orthopedics business earlier in the year. The system gives surgeons the tools to treat a wide range of end-stage ankle arthritis patients with varying anatomy and deformity by performing primary and revision surgeries. Implantation merely requires one simple cut of the talus. Implant and instrument design advancements and the streamlined surgical technique help minimize potential complications and address common ankle arthroplasty challenges.
“Our CADENCE total ankle replacement system was designed for a more predictable procedure,” said Lovato. “The recent launch of the CADENCE flat cut talus expands our continuum of care for patients with end-stage ankle arthritis. The addition allows surgeons to treat a wider range of patients with varying anatomy and deformity for primary and revision procedures, while simultaneously addressing the customer segment looking for efficiency in their surgical approach with one cut on the talus. The dedication to innovation and a focus on peer to peer education is driving a reduction in the learning curve for the foot and ankle surgeon community.”
Arthritis can also take a heavy toll on hands, causing pain, deformity, and disability. Unfortunately, surgery to repair the damage is rare because finger surgery has a high complication and failure rate. It can also sacrifice mobility for pain relief. The two main surgical options for hand arthritis are fusion and total knuckle replacement. Hand joint replacements, according to the Arthritis Foundation, can be less satisfactory than hip or knee replacements because hinged finger implants don’t fully replicate normal finger motion. Most are made from silicone, which is flexible but breaks and slips easily. Some studies have found up to 30 percent of silicone implants fail within 10 years.
Smith+Nephew also acquired Integra’s Ascension MCP (metacarpophalangeal) joint replacement implant earlier this year. Ascension MCP consists of separate proximal and distal components and a one-piece silicone spacer consisting of proximal and distal intramedullary stems and central flexible hinge. The proximal (metacarpal) component replaces the metacarpal head and the distal (phalangeal) replaces the proximal phalanx’s base. The implants come in six sizes, using color-coded instrumentation per intraoperative choice. The components are press fit, so they do not require cement. The company also acquired the Ascension PIP (proximal interphalangeal) total joint implant, NuGrip CMC (carpometacarpal—thumb) joint implant, PyroSphere CMC, and Pyrocarbon Lunate implant from Integra.
“We offer an alternative bearing material called Pyrocarbon in small joint replacements of the hand,” said Lovato. “Patented On-X Pyrocarbon is a form of carbon tailored for durability and biocompatibility. Decades of biomedical research and clinical outcomes have demonstrated Pyrocarbon is highly wear-resistant against both itself and native cartilage, and has shown favorable wear characteristics compared to metallic alloys and polymers—specifically in extremity joints. As a result, this unique material offers the ability to extend these devices’ functional longevity and lifetime.”
Supplier Insight
The skyrocketing growth of the extremity orthopedics market in recent years has trickled down to manufacturing partners as well. The industry’s rapid growth, like many others in the orthopedics industry, has necessitated the need for manufacturing partnerships with firms well-versed in making the necessary instruments and implants.
“The extremities segment has been growing at double digit rates for the past few years,” said Sophie Moulin, EMEA sales manager for Intech, a Rang-du-Fliers, France-based contract manufacturer of orthopedics instruments and implants. “Medical device companies had no other choice but to rely on outside partners to support their rapid growth and give patients life-enhancing surgical technologies. When selecting a supply base, medical device companies typically turn to partners with vertically integrated capabilities, and particularly for implants—from machining, to anodizing, to sterile packaging.”
Optimizing surgical instrument cases and trays improves efficiency and reduces cost. In addition to the substantial cost savings possible, tray optimization decreases tray weights and cleaning times without negatively impacting turnover times. Lean methodology boosts efficiency in instrument tray usage, and minimizes hospital cost while encouraging surgeon and staff participation through continuous process improvement.
“Extremity sets tend to be the most innovative from a cases and trays perspective,” said Moulin. “They’re comprised of typical instrument brackets often combined with various implant caddies and pivoting shelves/storage, or nifty retaining mechanisms that allow for an optimized layout. Other than screws and plates, an extremity system will typically include clamps, targeting devices, inserters/impactors, and reamers/drills.”
Manufacturers well-versed in making instruments for minimally invasive surgeries find that the translation to extremity orthopedic instruments is relatively simple.
“Because of our expertise in spine MIS (minimally invasive surgery) devices, we have always been a strong proponent to support their growth,” said Moulin. “The complexity of such MIS devices translates naturally into producing small and intricate shoulder and ankle replacement devices.”
“Historically, patients suffering with advanced wrist pain caused by arthritis or trauma were treated with older versions of total wrist arthroplasty systems or with wrist fusions,” Arnold-Peter C. Weiss, M.D., chief - Hand, Upper Extremity & Microvascular Surgery, vice chairman and professor of Orthopaedics, Warren Alpert Medical School, Brown University, told the press. “Existing total wrist arthroplasty implants on the market today may enable some wrist motion but are at higher risk of carpal loosening. Alternatively, a wrist fusion may severely limit a patient’s ability to move and use their wrist. Neither option restores wrist function, and that’s the core reason the WristMotion TWA System is a significant step forward for surgeons and patients.”
WristMotion TWA leverages over 12 years of data focused on stability and improving wrist implants with a proprietary taper post-fixation for long-term stability, and anatomic design for motion preservation. The modular joint preservation system replaces the radial and carpal sides of the wrist joint. A dual curvature carpal design feature combined with the dorsal flange allows for greater range of extension due to increased implant surface area, according to the company. Early clinical feedback on instrumentation, surgical technique, and post-op range of motion has been positive. “The WristMotion TWA System’s proprietary central threaded taper post helps ensure secure carpal fixation,” William B. Geissler, M.D., Alan E. Freeland Chair of Hand Surgery, professor and chief, Division of Hand and Upper Extremity, University of Mississippi, told the press. “This secure carpal fixation, coupled with the ability to adjust the height and tension of the wrist joint on the radial side rather than carpal, strengthens the implant’s stability and also preserves the natural center of rotation.” Geissler was the first surgeon in Mississippi to implant the WristMotion TWA System. “It is truly the next generation of total wrist arthroplasty.”
Moving up the ladder a bit, ODT Top 10 denizen DJO launched the MedShape DynaNail Hybrid Fusion System this past September. The system leverages active, adaptive healing technology to maintain dynamic compression at the fusion site with an anatomically friendly design that simplifies insertion. Its combined screw/nail design provides a balance of ease and reliability. A threaded, screw-like tip complements bone anatomy and the distal transverse calcaneal screw offers stability and prevents device migration. Maintained active compression is achieved via proprietary Internal Superelastic NiTiNOL Element, which responds to up to 5 mm of bone settling or resorption at the site of healing depending on the length of the implant.
“The DynaNail Hybrid provides the maintained active compression necessary for successful fusion in a package designed specifically to complement subtalar anatomy,” Dr. Samuel Adams of Duke University Medical Center told the press. “Not only can I implant with ease and efficiency, but the active, adaptive healing approach instills confidence that my patients will experience the best possible outcomes.”
According to Technavio, the extremities market is poised to grow to $3.24 billion, progressing at a CAGR of over 8 percent between 2021 and 2025. The extremity orthopedics market differs from the hip and knee sectors, where major players comprise a majority of the market. The market is fragmented, with several players occupying it, and is segmented by upper (shoulder, elbow, hand/wrist) and lower (foot/ankle) extremities. The upper extremities segment accrued maximum revenue, according to Technavio, due to the increasing incidence of work-related injuries and road accidents.
The North American market, according to the market research firm, will be responsible for 44 percent of growth this year. This was mainly driven by presence of favorable reimbursement programs such as the Comprehensive Care for Joint Replacement (CJR) Model, the Surgical Hip and Fracture Treatment (SHFFT) Model, and the Merit-based Incentive Payment System (MIPS) Model in the region.
Adoption of 3D printing and incorporation of robotics are increasing efficiency and reducing time required for surgeries, and customizing implants according to the patient’s anatomy. The technological advances are increasing extremities products adoption among end-users.
Although the market is quite fractured, this article will examine how two global orthopedic device manufacturers and one manufacturing partner are addressing the extremity orthopedics industry.
DePuy Synthes
Global orthopedic device manufacturer DePuy Synthes touts a wide variety of extremity products in its portfolio. Its upper extremities arsenal contains technologies to assist with shoulder, wrist, elbow, and clavicle fractures. The foot and ankle franchise targets foot, ankle, and calcaneal fractures, as well as hammertoe, hallux valgus, first MTP (metatarsophalangeal) fusion, midfoot arthrodesis, flatfoot reconstruction, and deformity correction. The company also harbors technologies to address shoulder repair and replacement.
“Extremities is a complex space spanning both upper and lower extremities that has tremendous potential to address multiple unmet needs for patients, surgeons, and providers,” said Oray Boston, worldwide president, DePuy Synthes TECA (Trauma, Extremities, Craniomaxillofacial and Animal Health). “We focus on innovation with the goal to improve clinical outcomes for patients and surgeons, while supporting providers’ needs in this rapidly evolving healthcare environment. We provide solutions, both with implants and surgical process perspectives, that have the potential to improve bone healing, increase surgical precision, increase intra-operative flexibility, and reduce post-operative complications.”
Elective surgery restrictions negatively impacted ambulatory surgical center (ASC) finances in early 2020, but the industry largely recovered rapidly and was poised to accept a greater share of procedures this year. Driven to minimize healthcare costs, insurers were already attempting to steer procedures to ASCs instead of costlier hospitals prior to the COVID-19 pandemic. Infection concerns due to the pandemic are making ASCs an even more popular choice for patients, as well. Becker’s ASC Review predicted more partnerships formed between existing surgery centers and hospitals and more joint-venture centers between independent practices and hospitals.
“As more and more extremities surgeries take place outside of large hospitals, we are putting specialized teams in place that are working to better understand the needs of customers to support sites of care such as ASCs,” said Boston. “Having deep understanding of their needs allows us to provide custom tailored solutions to meet their requirements.”
The company touts an extensive portfolio of extremity orthopedic products.
“Extremities is an area of high strategic importance where we have made significant investments, both in portfolio innovation and commercial infrastructure, said Boston. “Just in the past two years we have launched new headless compression screws, a radial head replacement system, DePuy Synthes Hammertoe Continuous Compression Implant, FIBULINK Syndesmosis Repair System, and the Variable Angle LCP Periprosthetic Proximal Femur Plating System.”
The company’s headless compression screws are self-drilling and self-tapping for surgeon-controlled compression and simplified technique. The smallest of the screws (1.5 mm) can be used for DIP arthrodesis, metacarpal base or head fracture, and Weil osteotomy (distal oblique osteotomy of the metatarsal head). The next sizes up (2.4 mm and 3 mm) are used for PIP arthrodesis, condylar fracture radial styloid fracture, and Chevron osteotomy.
“The headless compression screw system is the most comprehensive portfolio in its class on the market to address a wide range of surgical trauma and elective procedures,” said Boston. “This system offers titanium alloy, self-drilling/self-tapping screws that feature reverse cutting flutes, differential thread pitch and offer uncompromised precision through an innovative cutting edge for improved cutting efficiency.”
DePuy Synthes’ extramedullary implant for hammertoe fixation was designed with Nitinol, according to company, for continuous compression and superior rotational stability with greater distraction resistance. Designed for small bone reconstruction and fusion of the phalanges in toes, the extramedullary configuration provides bone-stock preservation and easy removal as well. The technology was added to the company’s portfolio from its acquisition of Biomedical Enterprises Inc. in 2016.
“The DePuy Synthes Hammertoe Continuous Compression Implant is the first extramedullary continuous compression implant on the market designed to provide greater stability, preservation of bone stock, and easier removal than intramedullary devices for the treatment of Hammertoe, which occurs as a result of a muscle imbalance that puts pressure on the toe tendons and joints,” said Boston. “Muscles work in pairs to straighten and bend the toes. If the toe is bent in one position long enough, the muscles and joints tighten and cannot stretch out.”
The firm’s radial head replacement system was launched last October for patients with destabilizing radial head fractures that can’t be repaired. It can also be used for elective management of elbow arthritis. The smooth, stemmed implants were designed to allow for unrestricted motion, allowing the stem to self-center inside the radial canal.
“The Radial Head Replacement System is used to treat radial head fractures and is the first with color-coded radiolucent trials that allow in-situ height determination and visualization of proximal ulna and joint,” said Boston. “It features a smooth stem design, which may enhance the implant’s ability to self-align and dial-in when the elbow goes through a range of motion and may articulate with the capitellum throughout the entire arc of motion.”
October 2020 saw the release of the company’s FIBULINK Syndesmosis Repair System to enable precise tension control and physiologic motion for patients with traumatic ankle injuries. The technology was acquired earlier in 2020 from Akros Medical. According to DePuy Synthes, its high-strength suture bridge eliminates complication risks associated with broken synesmotic screws. Syndesmotic fixation can be performed without a medial incision or hardware, minimizing medial side complications like neurovascular structure damage or soft tissue entrapment. The implants come in stainless steel or titanium and are compatible with the firm’s distal fibula plates and any plate hole that accepts a 4 mm non-locking cortex screw.
“The FIBULINK Syndesmosis Repair System is the only flexible syndesmotic repair system that has the ability to fine tune and readjust tension intraoperatively,” said Boston. “It eliminates medial soft tissue disruption and helps improve procedural efficiency by delivering fixation through a single lateral incision (compared to suture button constructs).”
The company’s 2.7 mm VA LCP (variable angle locking compression plate) Clavicle Plate System hit the market this past June, providing tools to treat lateral, shaft, and medial fractures for small, medium, and large clavicles. To develop the technology the company observed over 600 clavicle CT scans from a broad-based population of patients while analyzing 15 separate bone parameters to identify correlation between patient height and clavicle shape.
“The VA LCP Clavicle Plate System offers plate shapes that reflect the correlation between patient stature and clavicle size to match the clavicle’s bow and contour and accommodate a broad range of anatomic variability for the treatment of collarbone fractures. This new system offers thinner plates, a more accurate plate to bone fit, and reduced prominence.”
Smith+Nephew
Global medical device manufacturer Smith+Nephew also touts an impressive array of extremity orthopedic surgery products. These include a wide variety of small joint surgical instruments, suture anchors, hand and wrist joint fusion, repair, and replacement technologies, shoulder repair and replacement implants, and foot/ankle fixation, fusion, and joint replacement products.
“We offer advanced materials designed for durability and longevity in joint replacement surgery,” said Leigh Lovato, senior director of global marketing, Trauma & Extremities, for Smith+Nephew. “Orthopedic joint replacements historically are made out of traditional metallic alloys and polymers. Over time, metals can wear polymers, which may result in a shorter device lifespan.”
To perform ankle replacement surgery, the tibia and talus surface making up the tibiotalar joint is removed and metal components are inserted into the bone. A plastic insert is attached to the tibial metal piece. The metal piece on the talus will rub against the plastic piece attached to the tibial implant, removing ankle arthritis pain and preserving joint motion.
“Total ankle replacement is a historically complex procedure with a steep learning curve,” said Lovato. “Compared to large joint replacements such as hips and knees, procedural volume of ankle replacement procedures per year is relatively low. These factors drive the need for implant and instrument design simplicity to enable a more reproducible and predictable procedure with the goal of improving patient outcomes. Our goal is to satisfy this need through new product innovation and enabling peer-to-peer educational opportunities.”
The company rolled out the CADENCE Total Ankle Flat Cut Talar Dome System this past August. This total ankle replacement addition to Smith+Nephew’s portfolio was the result of acquiring Integra LifeScience’s extremity orthopedics business earlier in the year. The system gives surgeons the tools to treat a wide range of end-stage ankle arthritis patients with varying anatomy and deformity by performing primary and revision surgeries. Implantation merely requires one simple cut of the talus. Implant and instrument design advancements and the streamlined surgical technique help minimize potential complications and address common ankle arthroplasty challenges.
“Our CADENCE total ankle replacement system was designed for a more predictable procedure,” said Lovato. “The recent launch of the CADENCE flat cut talus expands our continuum of care for patients with end-stage ankle arthritis. The addition allows surgeons to treat a wider range of patients with varying anatomy and deformity for primary and revision procedures, while simultaneously addressing the customer segment looking for efficiency in their surgical approach with one cut on the talus. The dedication to innovation and a focus on peer to peer education is driving a reduction in the learning curve for the foot and ankle surgeon community.”
Arthritis can also take a heavy toll on hands, causing pain, deformity, and disability. Unfortunately, surgery to repair the damage is rare because finger surgery has a high complication and failure rate. It can also sacrifice mobility for pain relief. The two main surgical options for hand arthritis are fusion and total knuckle replacement. Hand joint replacements, according to the Arthritis Foundation, can be less satisfactory than hip or knee replacements because hinged finger implants don’t fully replicate normal finger motion. Most are made from silicone, which is flexible but breaks and slips easily. Some studies have found up to 30 percent of silicone implants fail within 10 years.
Smith+Nephew also acquired Integra’s Ascension MCP (metacarpophalangeal) joint replacement implant earlier this year. Ascension MCP consists of separate proximal and distal components and a one-piece silicone spacer consisting of proximal and distal intramedullary stems and central flexible hinge. The proximal (metacarpal) component replaces the metacarpal head and the distal (phalangeal) replaces the proximal phalanx’s base. The implants come in six sizes, using color-coded instrumentation per intraoperative choice. The components are press fit, so they do not require cement. The company also acquired the Ascension PIP (proximal interphalangeal) total joint implant, NuGrip CMC (carpometacarpal—thumb) joint implant, PyroSphere CMC, and Pyrocarbon Lunate implant from Integra.
“We offer an alternative bearing material called Pyrocarbon in small joint replacements of the hand,” said Lovato. “Patented On-X Pyrocarbon is a form of carbon tailored for durability and biocompatibility. Decades of biomedical research and clinical outcomes have demonstrated Pyrocarbon is highly wear-resistant against both itself and native cartilage, and has shown favorable wear characteristics compared to metallic alloys and polymers—specifically in extremity joints. As a result, this unique material offers the ability to extend these devices’ functional longevity and lifetime.”
Supplier Insight
The skyrocketing growth of the extremity orthopedics market in recent years has trickled down to manufacturing partners as well. The industry’s rapid growth, like many others in the orthopedics industry, has necessitated the need for manufacturing partnerships with firms well-versed in making the necessary instruments and implants.
“The extremities segment has been growing at double digit rates for the past few years,” said Sophie Moulin, EMEA sales manager for Intech, a Rang-du-Fliers, France-based contract manufacturer of orthopedics instruments and implants. “Medical device companies had no other choice but to rely on outside partners to support their rapid growth and give patients life-enhancing surgical technologies. When selecting a supply base, medical device companies typically turn to partners with vertically integrated capabilities, and particularly for implants—from machining, to anodizing, to sterile packaging.”
Optimizing surgical instrument cases and trays improves efficiency and reduces cost. In addition to the substantial cost savings possible, tray optimization decreases tray weights and cleaning times without negatively impacting turnover times. Lean methodology boosts efficiency in instrument tray usage, and minimizes hospital cost while encouraging surgeon and staff participation through continuous process improvement.
“Extremity sets tend to be the most innovative from a cases and trays perspective,” said Moulin. “They’re comprised of typical instrument brackets often combined with various implant caddies and pivoting shelves/storage, or nifty retaining mechanisms that allow for an optimized layout. Other than screws and plates, an extremity system will typically include clamps, targeting devices, inserters/impactors, and reamers/drills.”
Manufacturers well-versed in making instruments for minimally invasive surgeries find that the translation to extremity orthopedic instruments is relatively simple.
“Because of our expertise in spine MIS (minimally invasive surgery) devices, we have always been a strong proponent to support their growth,” said Moulin. “The complexity of such MIS devices translates naturally into producing small and intricate shoulder and ankle replacement devices.”