Sam Brusco, Associate Editor05.26.22
Imagine, for a moment, that you’ve just had knee replacement surgery. Your doctor fixed what was wrong, but you’ll initially still feel some pain and stiffness. Now that the surgical procedure is complete, it’s your turn to make sure the surgery is a success. That means working to restore the strength and mobility to your knees. The process is equally important as successful implantation of the new knee, and it’s not easy.
The rehabilitation process begins the day after surgery—you’ll be on your feet, probably using a cane, crutches, parallel bars, or a walker. You’ll meet with a physical therapist to guide you through a variety of exercises to improve movement and increase blood flow to the legs and feet. The therapist will show you how to get in and out of bed, and how to use crutches or the walker. A nurse or occupational therapist will teach you the best way to dress, bathe, and use the toilet.
After leaving the hospital, you might transfer to a dedicated rehab facility. In that case, a team of nurses, physical therapists, and occupational therapists, among others, will devise a program. Some patients decide to rehab at home, which, for many, is a good idea because a number of studies have shown those receiving physical therapy at home do as well as those staying in an in-patient facility.
The first TKR surgeries with Persona IQ took place last October at the Hospital for Special Surgery (HSS). The technology enables a doctor to actively monitor patient recovery from TKR with real-world, objective data to supplement care.
“Persona IQ is the world’s first and only smart knee implant that collects objective kinematic data like range of motion, step count, cadence, walking speed, stride length, and distance traveled directly from the knee implant,” Liane Teplitsky, global president of Robotics and Technology & Data Solutions, told ODT. “Combining the power of Zimmer Biomet’s proven and trusted Persona The Personalized Knee with Canary Medical’s proprietary implantable canturio te tibial extension sensor technology, Persona IQ works with mymobility, a digital care management platform. Persona IQ smart implant data is displayed in the mymobility Care Management Platform providing both surgeons/care teams and patients remote access to key post-operative metrics throughout the patient’s surgical journey to monitor activity levels between office visits during post-total knee arthroplasty (TKA) care, supplementing patient care.”
In the first few weeks after knee replacement, the patient is required to undergo a great deal of hard work to rehabilitate. There can be deviations in recovery that can set the patient on the wrong path. Using Persona IQ’s sensing capabilities, a patient who isn’t progressing as well as the doctor would like can be identified and intervention can be taken as necessary. That could mean a change in the physical therapy plan, more education, anti-inflammatory medication, or an ice machine. According to the company, the implant provides a direct view of patient-specific data for at least 10 years.
“Persona IQ enhances a patient’s connection with their physician and looks to give them confidence in their journey to recovery,” said Teplitsky. “By providing patients with access to their own mobility data, they can look to be more engaged in their recovery. Patients never need to think about tracking data manually—it simply happens automatically when they are home and in range of their base station connected to their home internet.”
Zimmer Biomet partnered with Apple to launch the mymobility remote care management system in October 2018. Using an iPhone or Apple Watch, the platform delivers continuous data and patient-reported feedback to facilitate care, outcomes, and satisfaction for surgical preparation and recovery. The platform provides education and engagement, surveys, check-ins, messaging, physiologic and gait metrics, video guided exercises and skeletal tracking, analytics, and telemedicine capability.
“mymobility is a digital care management platform that helps clinicians deliver support and guidance to patients through a connected care experience, while remotely monitoring patient progress by tracking Patient Reported Outcome Measures (PROMs), engagement and adherence to care plans, and providing virtual physical therapy,” said Teplitsky. “mymobility recently added a new Skeletal Tracking Shoulder Range of Motion (ROM) feature that allows patients with compatible smartphones to measure and track shoulder ROM and corresponding pain pre- and post-operatively throughout the episode of care. The feature collects ROM simply and easily through cameras on their phone, as opposed to only being collected by clinicians at in-person appointments typically assessed by eye.”
According to trial data released in November 2020, using the mymobility remote care management platform on Apple Watch and iPhone for TKR demonstrated similar early outcomes to traditional care models and required “significantly fewer” physical therapy visits (p<0.0001). Further, the mymobility cohort of patients reported fewer emergency department visits and lower hospital readmission rates.
“mymobility is a part of ZBEdge Connected Intelligence Suite, which seamlessly connects data from our smart devices (Persona IQ), digital care management platform (mymobility), surgical robotics (ROSA Robotics Platform), and more to deliver data-powered clinical insights across the patient journey, with the goal of enhancing the joint replacement surgical experience,” explained Teplitsky.
In March the company released WalkAI technology for ZBEdge, which helps identify patients with lagging gait speed following hip or knee surgery. WalkAI’s proprietary algorithm analyzes the patient’s mobility to produce a personalized daily prediction of gait speed three months after surgery. The daily prediction is then compared to anonymized, real-world ZBEdge database data.
WalkAI became available to mymobility users at the end of March.
“ZBEdge recently added powerful predictive analytic capabilities with WalkAI, a dynamic artificial intelligence (AI) model integrated with iPhone mymobility users,” said Teplitsky. “WalkAI identifies patients predicted to have a lower gait speed outcome at 90 days after hip or knee surgery with the goal of identifying when recovery may not be on track, and potentially helping surgeons mitigate or minimize relative poor outcomes. Physicians receive WalkAI notifications through the mymobility clinician dashboard.”
Available mymobility care plans include: total, partial, and revision knee; total and revision hip; total and reverse total shoulder; ACL; meniscal repair; meniscectomy; rotator cuff repair; shoulder instability; total ankle; cervical disc replacement, fusion, and non-fusion; and lumbar fusion and non-fusion.
“WalkAI is just the first step in demonstrating our unique capability to deliver actionable predictions by connecting real-world data and AI through ZBEdge products and experiences,” said Teplitsky. “We continue to advance our vision of creating a seamlessly connected suite of digital health and robotic technologies to deliver data-powered clinical insights throughout the surgical journey.”
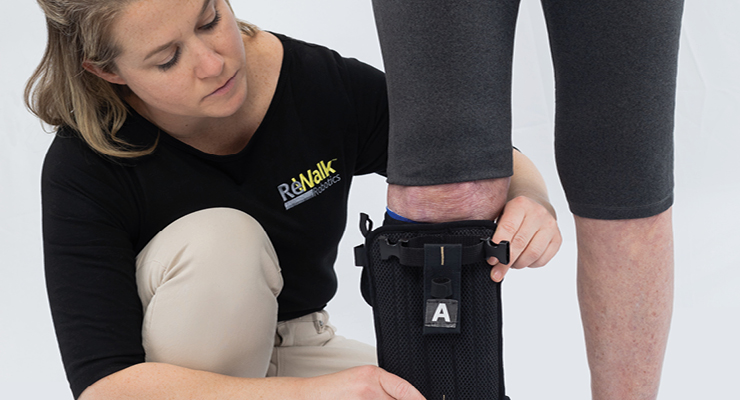
Israeli inventor Amit Goffer, Ph.D., became a quadriplegic after a 1997 ATV accident damaged his spinal cord. Through his own personal experience in using mobility devices for patients with spinal cord injury, he developed the first iteration of the ReWalk assistive exoskeleton device under the medical device company Argo Medical Technologies. The company does business as ReWalk Robotics in the U.S.
The first clinical trials for ReWalk began in 2009, and the FDA approved its use in the clinical setting in 2010. Since then the device has had several more iterations, each with more advanced assistive technology.
“The ReWalk Personal Exoskeleton uses bilateral powered hip and knee motion and adjustable ankle motion to enable wheelchair users with a spinal cord injury (SCI) to regain functional ambulation during activities of daily living (ADLs) in the home and community to improve overall activity levels and address health consequences associated with a sedentary lifestyle. Using the ReWalk Exoskeleton, device users can perform activities such as standing, sitting, walking, and changes of direction,” Kathleen O’Donnell, ReWalk’s VP of marketing and new business development, told ODT. “The specific actions are initiated through a combination of position changes, detected by a tilt-sensor, and input commands via a remote control worn on the wearer’s wrist.”
The company’s latest iteration of its wearable exoskeleton technology, the ReWalk Personal 6.0 Exoskeleton, earned FDA approval in 2014. It’s designed to be used all day at home and in the community. The battery-powered ReWalker has motors at the hip and knee joints, controlled via subtle changes in the wearer’s center of gravity. Tilting the upper body forward initiates sensing by the system, which begins the first step. Repeated shifting of the body creates a sequence of steps to mimic natural, functional gait of the legs.
Rehabilitation from a debilitating stroke is a long, arduous process. It’s different for each patient—it can take weeks, months, or even years to regain certain functions. Some patients recover fully, but others may have long-term or lifelong disabilities. Problems can include paralysis or weakness, cognitive issues, and pain or numbness, among others.
Stroke rehabilitation involves speech, physical, and occupational therapy. The physical therapy can be grueling, with exercises to help relearn movement and coordination skills lost because of the stroke.
To address this clinical need, ReWalk’s ReStore soft exo-suit for rehabilitation of lower limb disability due to stroke earned FDA clearance and CE mark approval 2019 to treat stroke survivors with mobility challenges. The technology was originally developed at Harvard University’s Wyss Institute for Biologically Inspired Engineering, where it also underwent clinical testing. ReWalk and the Wyss Institute had entered a multi-year collaboration agreement in 2016 providing the company access to future technologies emerging from the collaboration with relevance to further stroke products or other therapies.
“Our most recent product is the ReStore soft exo-suit for gait training of patients recovering from a stroke,” said O’ Donnell. “This fabric-based device provides targeted, adjustable levels of assistance to the ankle to help retrain improved walking function during ground clearance and push-off. This product can be used in physical therapy clinics to help therapists train patients to improve their walking speed, endurance, and overall walking function after a stroke.”
Two years later, ReWalk unveiled its powered orthotic exo-suit designed specifically for reduced ankle function as a result of stroke.
“In November we received Breakthrough Device designation for our ReBoot product, a lightweight, battery-powered exo-suit intended to assist ambulatory functions in individuals with reduced ankle function related to neurological injuries such as a stroke and it’s intended for home and community use,” said O’Donnell. “The ReBoot is a sister product to the ReStore Exo-Suit, which received FDA clearance in 2019 for use in the rehabilitation setting.”
ReBoot works with conjunction with affected leg muscles to assist with maintaining safe foot positioning and pushing off the ground to improve gait. ReBoot can also potentially help muscle reeducation, prevent or slow disuse atrophy, maintain or increase joint range of motion, improve walking speed and endurance without using the device, or reduce fall incidence. Its breakthrough status helps ReWalk more readily purse FDA approval, and at the time of the announcement the company was finalizing ReBoot’s design and development and preparing for clinical studies.
e-vive, CyMedica’s app-controlled, data-driven muscle activation therapy and patient engagement solution, won FDA clearance in January 2017. The tele-rehab solution for ACL and total knee replacement surgery patients consists of three components. A sensor-laden conductive garment captures and transmits range of motion data and steps while holding electrodes in place to deliver effective quadriceps activation via neuromuscular electrical stimulation (NMES). A controller monitors and controls the muscle stimulation.
The e-vive app, operable on any smartphone, collects data points and sends them to the cloud for physicians or physical therapists to view and analyze. Treatment is then adjusted (or kept the same) based on clinician review. In 2019, the firm also launched a 120-patient clinical trial to evaluate e-vive muscle strengthening and a patient engagement system that collects activity and health data by a commercially available smartwatch for knee osteoarthritis (OA) pain.
Knee OA, also known as degenerative joint disease, affects over 10 million Americans and costs the healthcare system tens of billions of dollars. Over half of people with knee OA are under 65 and may live for decades after their diagnosis. Its prevalence is expected to continue increasing due to aging, obesity, and sports injuries.
In 2021, the company earned FDA clearance for another therapy tool, IntelliHab, to manage knee osteoarthritis pain. The home-based therapeutic and digital health ecosystem provides a non-opioid, non-invasive neuromodulation treatment and connects providers to progress in real-time. The advancement was the company’s first approved solution to treat pain associated with knee arthritis.
“CyMedica’s unique, patented therapy has recently been clinically proven and FDA cleared to reduce knee pain associated with arthritis,” CyMedica Orthopedics president and CEO Rob Morocco told ODT. “We now offer patients at-home pain relief therapy earlier in their journey to a total knee replacement. Our primary goal has been to support patients during the rehabilitation period but our focus is now expanding to treat pain following an arthritis diagnosis, giving patients a tool to manage joint disease symptoms and maintain function until surgery is needed. The newly obtained pain indication lets physicians incorporate a non-invasive, non-narcotic modality to manage osteoarthritis, from early onset of disease to post-operative rehabilitation and beyond.”
IntelliHab’s clinical evidence demonstrated the ability to strengthen quadriceps muscles to decrease pain and improve function. The tool provides patients with another option in the menu of modalities to tackle the cycle of pain and stiffness caused by knee OA. The technology provides a bridge between conservative management to more invasive treatment options. Patients can treat pain comfortably at home while reducing exposure to opioids.
“One of our future initiatives is to broaden our reach to the patient population not yet ready for surgery but suffering from knee osteoarthritis pain," said Morocco. "We are exploring new avenues to provide access to our technology to the 14 million patients seeking pain relief and wishing to maintain an active lifestyle. We believe patients are becoming more informed of pain relief and wellness treatment options available to them through direct channels.”
The rehabilitation process begins the day after surgery—you’ll be on your feet, probably using a cane, crutches, parallel bars, or a walker. You’ll meet with a physical therapist to guide you through a variety of exercises to improve movement and increase blood flow to the legs and feet. The therapist will show you how to get in and out of bed, and how to use crutches or the walker. A nurse or occupational therapist will teach you the best way to dress, bathe, and use the toilet.
After leaving the hospital, you might transfer to a dedicated rehab facility. In that case, a team of nurses, physical therapists, and occupational therapists, among others, will devise a program. Some patients decide to rehab at home, which, for many, is a good idea because a number of studies have shown those receiving physical therapy at home do as well as those staying in an in-patient facility.
Total Knee Replacement
There is a myriad of technologies to assist with the recovery and rehabilitation process. Global orthopedic device manufacturer Zimmer Biomet and Canary Medical, a medical data company aiming to improve healthcare outcomes with its smart implantable devices, earned de novo clearance from the U.S. Food and Drug Administration (FDA) in August 2021 for the tibial extension for its Persona IQ total knee replacement implant. The knee implant is outfitted with implantable tibial extension sensors that measure and determine range of motion, step count, walking speed, and other gait metrics.The first TKR surgeries with Persona IQ took place last October at the Hospital for Special Surgery (HSS). The technology enables a doctor to actively monitor patient recovery from TKR with real-world, objective data to supplement care.
“Persona IQ is the world’s first and only smart knee implant that collects objective kinematic data like range of motion, step count, cadence, walking speed, stride length, and distance traveled directly from the knee implant,” Liane Teplitsky, global president of Robotics and Technology & Data Solutions, told ODT. “Combining the power of Zimmer Biomet’s proven and trusted Persona The Personalized Knee with Canary Medical’s proprietary implantable canturio te tibial extension sensor technology, Persona IQ works with mymobility, a digital care management platform. Persona IQ smart implant data is displayed in the mymobility Care Management Platform providing both surgeons/care teams and patients remote access to key post-operative metrics throughout the patient’s surgical journey to monitor activity levels between office visits during post-total knee arthroplasty (TKA) care, supplementing patient care.”
In the first few weeks after knee replacement, the patient is required to undergo a great deal of hard work to rehabilitate. There can be deviations in recovery that can set the patient on the wrong path. Using Persona IQ’s sensing capabilities, a patient who isn’t progressing as well as the doctor would like can be identified and intervention can be taken as necessary. That could mean a change in the physical therapy plan, more education, anti-inflammatory medication, or an ice machine. According to the company, the implant provides a direct view of patient-specific data for at least 10 years.
“Persona IQ enhances a patient’s connection with their physician and looks to give them confidence in their journey to recovery,” said Teplitsky. “By providing patients with access to their own mobility data, they can look to be more engaged in their recovery. Patients never need to think about tracking data manually—it simply happens automatically when they are home and in range of their base station connected to their home internet.”
Zimmer Biomet partnered with Apple to launch the mymobility remote care management system in October 2018. Using an iPhone or Apple Watch, the platform delivers continuous data and patient-reported feedback to facilitate care, outcomes, and satisfaction for surgical preparation and recovery. The platform provides education and engagement, surveys, check-ins, messaging, physiologic and gait metrics, video guided exercises and skeletal tracking, analytics, and telemedicine capability.
“mymobility is a digital care management platform that helps clinicians deliver support and guidance to patients through a connected care experience, while remotely monitoring patient progress by tracking Patient Reported Outcome Measures (PROMs), engagement and adherence to care plans, and providing virtual physical therapy,” said Teplitsky. “mymobility recently added a new Skeletal Tracking Shoulder Range of Motion (ROM) feature that allows patients with compatible smartphones to measure and track shoulder ROM and corresponding pain pre- and post-operatively throughout the episode of care. The feature collects ROM simply and easily through cameras on their phone, as opposed to only being collected by clinicians at in-person appointments typically assessed by eye.”
According to trial data released in November 2020, using the mymobility remote care management platform on Apple Watch and iPhone for TKR demonstrated similar early outcomes to traditional care models and required “significantly fewer” physical therapy visits (p<0.0001). Further, the mymobility cohort of patients reported fewer emergency department visits and lower hospital readmission rates.
“mymobility is a part of ZBEdge Connected Intelligence Suite, which seamlessly connects data from our smart devices (Persona IQ), digital care management platform (mymobility), surgical robotics (ROSA Robotics Platform), and more to deliver data-powered clinical insights across the patient journey, with the goal of enhancing the joint replacement surgical experience,” explained Teplitsky.
In March the company released WalkAI technology for ZBEdge, which helps identify patients with lagging gait speed following hip or knee surgery. WalkAI’s proprietary algorithm analyzes the patient’s mobility to produce a personalized daily prediction of gait speed three months after surgery. The daily prediction is then compared to anonymized, real-world ZBEdge database data.
WalkAI became available to mymobility users at the end of March.
“ZBEdge recently added powerful predictive analytic capabilities with WalkAI, a dynamic artificial intelligence (AI) model integrated with iPhone mymobility users,” said Teplitsky. “WalkAI identifies patients predicted to have a lower gait speed outcome at 90 days after hip or knee surgery with the goal of identifying when recovery may not be on track, and potentially helping surgeons mitigate or minimize relative poor outcomes. Physicians receive WalkAI notifications through the mymobility clinician dashboard.”
Available mymobility care plans include: total, partial, and revision knee; total and revision hip; total and reverse total shoulder; ACL; meniscal repair; meniscectomy; rotator cuff repair; shoulder instability; total ankle; cervical disc replacement, fusion, and non-fusion; and lumbar fusion and non-fusion.
“WalkAI is just the first step in demonstrating our unique capability to deliver actionable predictions by connecting real-world data and AI through ZBEdge products and experiences,” said Teplitsky. “We continue to advance our vision of creating a seamlessly connected suite of digital health and robotic technologies to deliver data-powered clinical insights throughout the surgical journey.”
Spinal Cord Injury & Stroke
After a spinal cord injury, rehabilitation will be needed to optimize recovery and potentially adapt to a new way of life. Scientists are optimistic that research advances will someday make repairing spinal cord injuries possible. Research studies around the world are ongoing—in the meantime, treatments and rehabilitation allow many spinal cord injury patients to lead productive and independent lives.Israeli inventor Amit Goffer, Ph.D., became a quadriplegic after a 1997 ATV accident damaged his spinal cord. Through his own personal experience in using mobility devices for patients with spinal cord injury, he developed the first iteration of the ReWalk assistive exoskeleton device under the medical device company Argo Medical Technologies. The company does business as ReWalk Robotics in the U.S.
The first clinical trials for ReWalk began in 2009, and the FDA approved its use in the clinical setting in 2010. Since then the device has had several more iterations, each with more advanced assistive technology.
“The ReWalk Personal Exoskeleton uses bilateral powered hip and knee motion and adjustable ankle motion to enable wheelchair users with a spinal cord injury (SCI) to regain functional ambulation during activities of daily living (ADLs) in the home and community to improve overall activity levels and address health consequences associated with a sedentary lifestyle. Using the ReWalk Exoskeleton, device users can perform activities such as standing, sitting, walking, and changes of direction,” Kathleen O’Donnell, ReWalk’s VP of marketing and new business development, told ODT. “The specific actions are initiated through a combination of position changes, detected by a tilt-sensor, and input commands via a remote control worn on the wearer’s wrist.”
The company’s latest iteration of its wearable exoskeleton technology, the ReWalk Personal 6.0 Exoskeleton, earned FDA approval in 2014. It’s designed to be used all day at home and in the community. The battery-powered ReWalker has motors at the hip and knee joints, controlled via subtle changes in the wearer’s center of gravity. Tilting the upper body forward initiates sensing by the system, which begins the first step. Repeated shifting of the body creates a sequence of steps to mimic natural, functional gait of the legs.
Rehabilitation from a debilitating stroke is a long, arduous process. It’s different for each patient—it can take weeks, months, or even years to regain certain functions. Some patients recover fully, but others may have long-term or lifelong disabilities. Problems can include paralysis or weakness, cognitive issues, and pain or numbness, among others.
Stroke rehabilitation involves speech, physical, and occupational therapy. The physical therapy can be grueling, with exercises to help relearn movement and coordination skills lost because of the stroke.
To address this clinical need, ReWalk’s ReStore soft exo-suit for rehabilitation of lower limb disability due to stroke earned FDA clearance and CE mark approval 2019 to treat stroke survivors with mobility challenges. The technology was originally developed at Harvard University’s Wyss Institute for Biologically Inspired Engineering, where it also underwent clinical testing. ReWalk and the Wyss Institute had entered a multi-year collaboration agreement in 2016 providing the company access to future technologies emerging from the collaboration with relevance to further stroke products or other therapies.
“Our most recent product is the ReStore soft exo-suit for gait training of patients recovering from a stroke,” said O’ Donnell. “This fabric-based device provides targeted, adjustable levels of assistance to the ankle to help retrain improved walking function during ground clearance and push-off. This product can be used in physical therapy clinics to help therapists train patients to improve their walking speed, endurance, and overall walking function after a stroke.”
Two years later, ReWalk unveiled its powered orthotic exo-suit designed specifically for reduced ankle function as a result of stroke.
“In November we received Breakthrough Device designation for our ReBoot product, a lightweight, battery-powered exo-suit intended to assist ambulatory functions in individuals with reduced ankle function related to neurological injuries such as a stroke and it’s intended for home and community use,” said O’Donnell. “The ReBoot is a sister product to the ReStore Exo-Suit, which received FDA clearance in 2019 for use in the rehabilitation setting.”
ReBoot works with conjunction with affected leg muscles to assist with maintaining safe foot positioning and pushing off the ground to improve gait. ReBoot can also potentially help muscle reeducation, prevent or slow disuse atrophy, maintain or increase joint range of motion, improve walking speed and endurance without using the device, or reduce fall incidence. Its breakthrough status helps ReWalk more readily purse FDA approval, and at the time of the announcement the company was finalizing ReBoot’s design and development and preparing for clinical studies.
Osteoarthritis
Tempe, Ariz.-based CyMedica Orthopedics was founded in January 2013 by a prominent orthopedic surgeon and medical device entrepreneurs aiming to deliver faster, more predictable patient outcomes. The company developed a therapy-based, digital health technology platform to treat muscles weakened by knee osteoarthritis or surgery.e-vive, CyMedica’s app-controlled, data-driven muscle activation therapy and patient engagement solution, won FDA clearance in January 2017. The tele-rehab solution for ACL and total knee replacement surgery patients consists of three components. A sensor-laden conductive garment captures and transmits range of motion data and steps while holding electrodes in place to deliver effective quadriceps activation via neuromuscular electrical stimulation (NMES). A controller monitors and controls the muscle stimulation.
The e-vive app, operable on any smartphone, collects data points and sends them to the cloud for physicians or physical therapists to view and analyze. Treatment is then adjusted (or kept the same) based on clinician review. In 2019, the firm also launched a 120-patient clinical trial to evaluate e-vive muscle strengthening and a patient engagement system that collects activity and health data by a commercially available smartwatch for knee osteoarthritis (OA) pain.
Knee OA, also known as degenerative joint disease, affects over 10 million Americans and costs the healthcare system tens of billions of dollars. Over half of people with knee OA are under 65 and may live for decades after their diagnosis. Its prevalence is expected to continue increasing due to aging, obesity, and sports injuries.
In 2021, the company earned FDA clearance for another therapy tool, IntelliHab, to manage knee osteoarthritis pain. The home-based therapeutic and digital health ecosystem provides a non-opioid, non-invasive neuromodulation treatment and connects providers to progress in real-time. The advancement was the company’s first approved solution to treat pain associated with knee arthritis.
“CyMedica’s unique, patented therapy has recently been clinically proven and FDA cleared to reduce knee pain associated with arthritis,” CyMedica Orthopedics president and CEO Rob Morocco told ODT. “We now offer patients at-home pain relief therapy earlier in their journey to a total knee replacement. Our primary goal has been to support patients during the rehabilitation period but our focus is now expanding to treat pain following an arthritis diagnosis, giving patients a tool to manage joint disease symptoms and maintain function until surgery is needed. The newly obtained pain indication lets physicians incorporate a non-invasive, non-narcotic modality to manage osteoarthritis, from early onset of disease to post-operative rehabilitation and beyond.”
IntelliHab’s clinical evidence demonstrated the ability to strengthen quadriceps muscles to decrease pain and improve function. The tool provides patients with another option in the menu of modalities to tackle the cycle of pain and stiffness caused by knee OA. The technology provides a bridge between conservative management to more invasive treatment options. Patients can treat pain comfortably at home while reducing exposure to opioids.
“One of our future initiatives is to broaden our reach to the patient population not yet ready for surgery but suffering from knee osteoarthritis pain," said Morocco. "We are exploring new avenues to provide access to our technology to the 14 million patients seeking pain relief and wishing to maintain an active lifestyle. We believe patients are becoming more informed of pain relief and wellness treatment options available to them through direct channels.”