Business Wire11.17.16
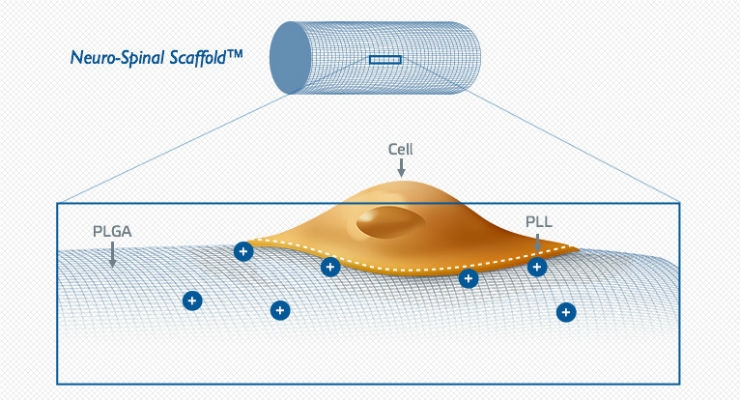
InVivo Therapeutics Holdings Corp. (NVIV) today announced that a new patient has been enrolled into The INSPIRE Study (InVivo Study of Probable Benefit of the Neuro-Spinal Scaffold for Safety and Neurologic Recovery in Subjects with Complete Thoracic AIS A Spinal Cord Injury) at Oregon Health & Science University (OHSU) in Portland, Oregon. Ahmed M. Raslan, M.D. and Jason J Chang, M.D., Assistant Professors of Neurological Surgery and study investigators, performed surgery and the implantation on the T9 neurologically complete patient approximately 36 hours after the injury occurred.
Mark Perrin, InVivo’s CEO and Chairman, said, “We are pleased that the patient is doing well and wish them continued recovery. We have nine patients enrolled and in follow up and, because we now have over 25 INSPIRE sites open, expect enrollment to increase in the coming months.”
For more information, please visit the company’s ClinicalTrials.gov registration site.