Business Wire01.25.17
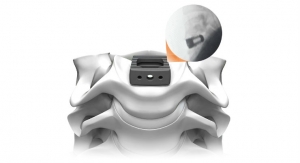
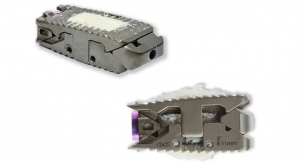
IMPLANET, a medical technology company specializing in vertebral and knee-surgery implants, has been given the green light by the American and European authorities, through FDA 510k clearance and CE marking, to market its new Jazz Frame implant.
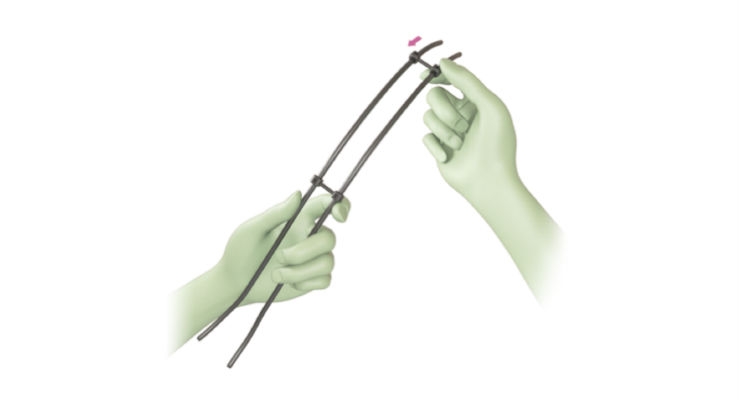
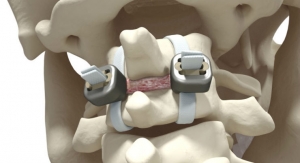
JAZZ Frame is a system of connectors, the final link in the JAZZ Band technological platform dedicated to the hybrid surgical technique. Implanet now offers surgeons the possibility of defining and optimizing a global strategy to reduce major deformities, thus maximizing long-term clinical outcomes.
“Since we have been using sublaminar braid implants to reduce and stabilize scoliotic deformities, we have been able to show significantly greater reductions than we previously obtained with our all-screw or hook-and-screw assemblies,” said Professor Keyvan Mazda, MD, Ph.D, Robert Debré Hospital, APHP, adding: “Using the JAZZ Frame facilitates the restoration of both frontal and sagittal balance, thanks to the simultaneous reduction of both thoracic curves. This is most notable in the case of the most complex deformities where shoulder imbalance is common. The result of years of clinical experience and of close collaboration with Implanet, JAZZ Frame allows us to be even more efficient and quick for the sole benefit of patients.”
Ludovic Lastennet, CEO of Implanet, added: “We continue to strictly adhere to, and execute our business plan. The rapid marketing clearance in Europe and the United States is a real source of satisfaction, innovation that maximizes the clinical value of our technology. Optimized for implementation of the “frame” technique, we expect this implant to be rapidly adopted by our partner surgeons, pediatric and adult deformity specialists alike. The marketing release of JAZZ Frame in our various markets is scheduled for the first quarter of 2017.”
JAZZ Frame is a system of connectors, the final link in the JAZZ Band technological platform dedicated to the hybrid surgical technique. Implanet now offers surgeons the possibility of defining and optimizing a global strategy to reduce major deformities, thus maximizing long-term clinical outcomes.
“Since we have been using sublaminar braid implants to reduce and stabilize scoliotic deformities, we have been able to show significantly greater reductions than we previously obtained with our all-screw or hook-and-screw assemblies,” said Professor Keyvan Mazda, MD, Ph.D, Robert Debré Hospital, APHP, adding: “Using the JAZZ Frame facilitates the restoration of both frontal and sagittal balance, thanks to the simultaneous reduction of both thoracic curves. This is most notable in the case of the most complex deformities where shoulder imbalance is common. The result of years of clinical experience and of close collaboration with Implanet, JAZZ Frame allows us to be even more efficient and quick for the sole benefit of patients.”
Ludovic Lastennet, CEO of Implanet, added: “We continue to strictly adhere to, and execute our business plan. The rapid marketing clearance in Europe and the United States is a real source of satisfaction, innovation that maximizes the clinical value of our technology. Optimized for implementation of the “frame” technique, we expect this implant to be rapidly adopted by our partner surgeons, pediatric and adult deformity specialists alike. The marketing release of JAZZ Frame in our various markets is scheduled for the first quarter of 2017.”