Houston Methodist01.10.18
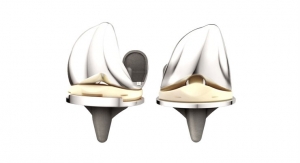
More than 1 million people undergo total joint replacements each year, and nearly 10,000 will develop infections. To reduce this infection risk, a Houston Methodist orthopedic surgeon created small antibiotic beads that are implanted with the new joint to slowly release medicine for several weeks.
Terry Clyburn, M.D., developed antibiotic microspheres that are as small as grains of salt and designed to release antibiotics at a level high enough to kill infections for at least three to six weeks—the timeframe when an infection is most likely to develop.
“There is a risk of infection with any surgery, but infections after a joint replacement surgery are harder to treat,” Clyburn said. “The metal implants are not connected to the body’s bloodstream, so the white blood cells sent to fight the infection cannot reach the implant and kill the bacteria.”
Currently, most joint replacement patients will be given intravenous antibiotics before and after their surgery to help stave off an infection. By coating the implant in the antibiotic microspheres before placing it in the patient’s joint, the antibiotics are delivered directly to the surgical site to help prevent bacteria from developing into an infection.
“Most products labeled as time release will release all of their medication as soon as the protective outer layer is dissolved,” Clyburn said. “But these microspheres are truly time release as they have several layers of antibiotics that slowly dissolve and release the medication on the implant and surrounding tissues. Within six weeks, the microspheres are completely dissolved and leave nothing behind in the joint that could lead to future problems for the patient.”
In studies to determine the efficacy of the microspheres, Clyburn and his team contaminated two metal implants with staphylococcus aureus bacteria and coated one in the microspheres before inserting them in animal models. Zero infections developed in the model that received the microspheres.
“If an infection does develop after a joint replacement surgery, many patients may require surgery to thoroughly clean around the implant or even replace it,” Clyburn said. “That’s why I’ve been working for more than 15 years to develop these microspheres—to help protect my patients and reduce their risk for major complications after surgery.”
Clyburn developed the microspheres in collaboration with Rice University’s Antonios Mikos, Ph.D., and Catherine Ambrose, Ph.D., from UTHealth. He is currently seeking FDA approval of the antibiotic microspheres and estimates they will be in use in three to eight years.
Terry Clyburn, M.D., developed antibiotic microspheres that are as small as grains of salt and designed to release antibiotics at a level high enough to kill infections for at least three to six weeks—the timeframe when an infection is most likely to develop.
“There is a risk of infection with any surgery, but infections after a joint replacement surgery are harder to treat,” Clyburn said. “The metal implants are not connected to the body’s bloodstream, so the white blood cells sent to fight the infection cannot reach the implant and kill the bacteria.”
Currently, most joint replacement patients will be given intravenous antibiotics before and after their surgery to help stave off an infection. By coating the implant in the antibiotic microspheres before placing it in the patient’s joint, the antibiotics are delivered directly to the surgical site to help prevent bacteria from developing into an infection.
“Most products labeled as time release will release all of their medication as soon as the protective outer layer is dissolved,” Clyburn said. “But these microspheres are truly time release as they have several layers of antibiotics that slowly dissolve and release the medication on the implant and surrounding tissues. Within six weeks, the microspheres are completely dissolved and leave nothing behind in the joint that could lead to future problems for the patient.”
In studies to determine the efficacy of the microspheres, Clyburn and his team contaminated two metal implants with staphylococcus aureus bacteria and coated one in the microspheres before inserting them in animal models. Zero infections developed in the model that received the microspheres.
“If an infection does develop after a joint replacement surgery, many patients may require surgery to thoroughly clean around the implant or even replace it,” Clyburn said. “That’s why I’ve been working for more than 15 years to develop these microspheres—to help protect my patients and reduce their risk for major complications after surgery.”
Clyburn developed the microspheres in collaboration with Rice University’s Antonios Mikos, Ph.D., and Catherine Ambrose, Ph.D., from UTHealth. He is currently seeking FDA approval of the antibiotic microspheres and estimates they will be in use in three to eight years.