Miach Orthopaedics Inc.01.14.22
Miach Orthopaedics Inc., a privately held company dedicated to developing bio-engineered surgical implants for connective tissue restoration, announced the first patient has been treated in the company’s BEAR III study of the BEAR Implant—the first medical technology to clinically demonstrate that it enables healing of a patient's torn anterior cruciate ligament (ACL). Miach initiated BEAR III to continue to build upon the extensive base of evidence for the BEAR Implant, which received De Novo approval from the U.S. Food and Drug Administration (FDA) in December 2020.
BEAR III is a single-arm, multi center cohort study enrolling 250 patients, all of whom will receive the BEAR Implant and will be followed for 10 years. The first patient, a 32-year-old male with a mid-substance ACL tear, was treated at MedStar Surgery Center at Timonium, an outpatient site affiliated with Medstar Union Memorial Hospital in Baltimore, Md.
“The BEAR Implant is a promising new technology that could improve the way ACL tears are treated,” said Dr. Richard Levine, the orthopedic surgeon who performed the surgery. “The BEAR Implant offers patients a treatment option to restore their ACL instead of reconstructing it with a graft.”
The BEAR III Trial for Bridge-Enhanced ACL (Anterior Cruciate Ligament) Restoration is enrolling patients at orthopedic centers in Georgia, Louisiana, Maryland, Massachusetts, New Jersey, Rhode Island, and South Dakota.
“We have seen consistently positive results in the BEAR I and BEAR II studies,” said Martha Shadan, president and CEO, Miach Orthopaedics. “We’re excited to have some of the leading orthopedic centers across the U.S. partnering with us on BEAR III as we continue to add to our extensive base of clinical evidence for the BEAR Implant.”
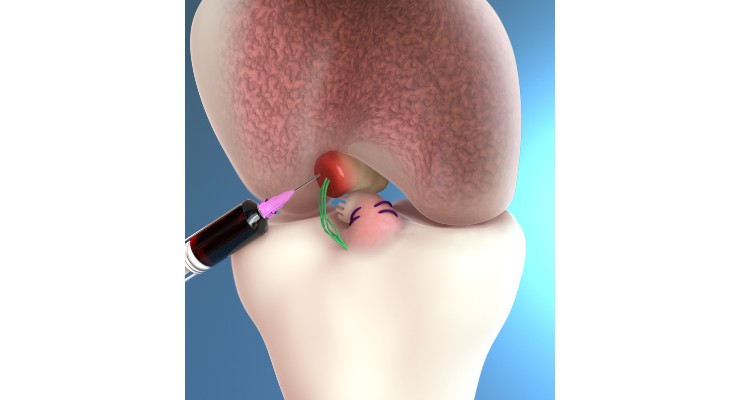
The Bridge-Enhanced ACL Restoration (BEAR) Implant is a proprietary bio-engineered implant used to facilitate healing of the torn ACL. Unlike reconstruction, which is the current standard of care, the BEAR Implant does not require a second surgical wound site to remove a healthy tendon from another part of the leg or the use of a donor tendon. The BEAR Implant acts as a bridge between the two ends of the torn ACL. The surgeon injects a small amount of the patient’s own blood into the implant and inserts it between the torn ends of the ACL in a minimally invasive procedure. The combination of the BEAR Implant and the patient’s blood enables the body to heal the torn ends of the ACL back together while maintaining the ACL’s original attachments to the femur and tibia. The BEAR Implant is absorbed by the body as the ACL heals.
The BEAR Implant is indicated for skeletally mature patients at least 14 years of age with a complete rupture of the ACL, as confirmed by MRI. Patients must have an ACL stump attached to the tibia to facilitate the restoration. The BEAR device must be implanted within 50 days of injury.
Miach Orthopaedics, located in Westborough, Mass., dedicated to developing bio-engineered surgical implants for connective tissue restoration. The company’s initial focus is the Bridge-Enhanced ACL Restoration (BEAR) Implant, which represents a paradigm shift in the treatment of ACL tears from reconstruction to restoration. The BEAR technology was pioneered by Martha Murray, M.D., founder of Miach Orthopaedics, at the Boston Children’s Hospital Department of Orthopaedic Surgery, with initial research funding provided by the NFL Players Association, Boston Children’s Hospital, and the National Institutes of Health.
BEAR III is a single-arm, multi center cohort study enrolling 250 patients, all of whom will receive the BEAR Implant and will be followed for 10 years. The first patient, a 32-year-old male with a mid-substance ACL tear, was treated at MedStar Surgery Center at Timonium, an outpatient site affiliated with Medstar Union Memorial Hospital in Baltimore, Md.
“The BEAR Implant is a promising new technology that could improve the way ACL tears are treated,” said Dr. Richard Levine, the orthopedic surgeon who performed the surgery. “The BEAR Implant offers patients a treatment option to restore their ACL instead of reconstructing it with a graft.”
The BEAR III Trial for Bridge-Enhanced ACL (Anterior Cruciate Ligament) Restoration is enrolling patients at orthopedic centers in Georgia, Louisiana, Maryland, Massachusetts, New Jersey, Rhode Island, and South Dakota.
“We have seen consistently positive results in the BEAR I and BEAR II studies,” said Martha Shadan, president and CEO, Miach Orthopaedics. “We’re excited to have some of the leading orthopedic centers across the U.S. partnering with us on BEAR III as we continue to add to our extensive base of clinical evidence for the BEAR Implant.”
The Bridge-Enhanced ACL Restoration (BEAR) Implant is a proprietary bio-engineered implant used to facilitate healing of the torn ACL. Unlike reconstruction, which is the current standard of care, the BEAR Implant does not require a second surgical wound site to remove a healthy tendon from another part of the leg or the use of a donor tendon. The BEAR Implant acts as a bridge between the two ends of the torn ACL. The surgeon injects a small amount of the patient’s own blood into the implant and inserts it between the torn ends of the ACL in a minimally invasive procedure. The combination of the BEAR Implant and the patient’s blood enables the body to heal the torn ends of the ACL back together while maintaining the ACL’s original attachments to the femur and tibia. The BEAR Implant is absorbed by the body as the ACL heals.
The BEAR Implant is indicated for skeletally mature patients at least 14 years of age with a complete rupture of the ACL, as confirmed by MRI. Patients must have an ACL stump attached to the tibia to facilitate the restoration. The BEAR device must be implanted within 50 days of injury.
Miach Orthopaedics, located in Westborough, Mass., dedicated to developing bio-engineered surgical implants for connective tissue restoration. The company’s initial focus is the Bridge-Enhanced ACL Restoration (BEAR) Implant, which represents a paradigm shift in the treatment of ACL tears from reconstruction to restoration. The BEAR technology was pioneered by Martha Murray, M.D., founder of Miach Orthopaedics, at the Boston Children’s Hospital Department of Orthopaedic Surgery, with initial research funding provided by the NFL Players Association, Boston Children’s Hospital, and the National Institutes of Health.