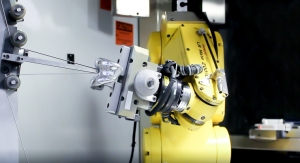
Jim Stertz, Vice President of Automation/Technology, Lowell Inc.09.15.20
As product development shifts from engineering to production, manufacturers take the lead on delivering parts that meet the engineer’s design intent and are on time and budget.
While not always visible to the customer, one way manufacturers can meet these expectations is through automated machining processes. Automation opens new ways to create a more consistent part at the point of manufacturing. It can also help manufacturers add capacity in their existing shop footprint and reduce the labor hours per part to positively deliver on lead times and cost.
This article offers a closer look at how automation may work behind the scenes to improve quality, delivery, and cost.
Automation for Quality: More Consistent Results
Automation can be applied at various points in manufacturing, and its application to machine tools can lead to quality improvements.
A component’s machining process will vary depending on the machine, tooling, material, or environment. The machinist needs to be familiar with all aspects of the process to stay ahead of any potential drift in a feature’s size or location. They also need to be able to correctly offset the machine program to ensure ongoing conformance.
The number of variables in the process can be staggering. On the simpler end, a part may require 10 tools and have 20 features and dimensions to track. Some of the most complex parts may require 50 tools and 100 features and dimensions. This quickly escalates the complexity of managing the machining process and introduces additional opportunities for error.
Automating in-machine measurement can help across the range of parts. One way this happens is through in-machine probes. These probes measure tool cuts and dimensions during production at the machine level, and the software tracks which cuts and dimensions are made by which tools. The machine program reads the measured results and software conditional statements decide to continue the process, make tool offsets, or stop the activity.
The analysis may show any number of results, depending on the programming. Three main categories of results include:
Automation for Delivery: Additional Capacity Extends Production Limits
Another way automation drives results for device companies is in delivery and lead times. When contract manufacturers automate machines, it can often lead to around-the-clock machining. This can increase capacity within an existing facility.
As an example, a non-automated machine may run 50 to 100 hours per week, depending on the number of orders and shifts. Typically, when machinists aren’t around, the machine isn’t running.
Since automation is monitored by the machine control, a machine’s production capacity may be extended to up to 24 hours a day. A machine previously running at 50 to 100 hours per week may now be able to run 150 to 168 hours a week. This allows the manufacturer to produce more parts each week, shortening the overall lead time.
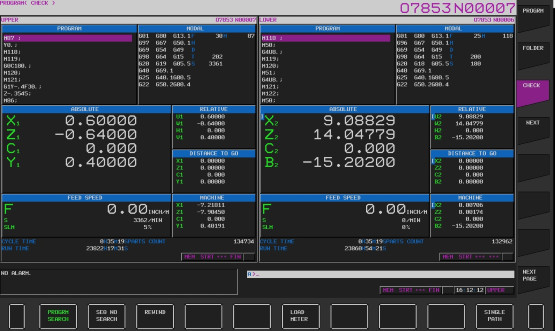
Machine control systems are programmed to read measurement results, and can be accessed remotely as seen here. Based on results, software conditional statements decide whether to continue the process, make tool offsets, or stop the process.
Automation for Cost: Around-the-Clock Production
Labor hours are the most expensive component of machining. Automation allows fewer people to manage the machining process, reducing the labor hours required for each part. This may lead to customer savings, because what used to require multiple people can now be managed by fewer machinists. It also enables domestic manufacturers to level the playing field compared to off-shore sources, and keep in balance the rising costs of doing business.
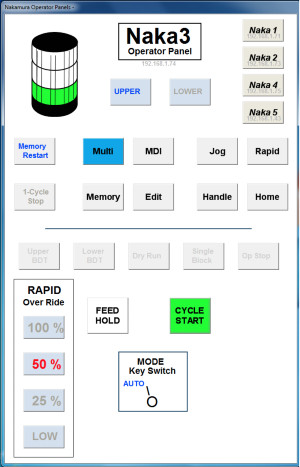
Operator panels give machinists control over the manufacturing process as they monitor production, and are accessible remotely as seen here.
Additional savings comes from automation’s ability to scale and flex based on demand. Rather than have more machinists work more shifts to scale up production, automation’s ability to run around the clock without machinist intervention again can help reduce labor hours. Employees shouldn’t have to work nights or weekends. Automation can allow machines to run nights and weekends with minimal machinist monitoring or intervention. When that extra capacity isn’t needed, the automation can be scaled back.
This doesn’t necessarily mean automation replaces machinist jobs. In the best scenarios, it means fewer hands have to be involved to accomplish work, and machinists are able to accomplish more complex tasks in their day. Additionally, improved quality, shorter lead times, and lower labor hours per part machined should allow a manufacturer to be more competitive, which may result in a higher volume of work for the machining team.
With automation, if something changes in the process, remote monitoring and alerts from the machine control can notify the machinist of an issue even when they aren’t in the building. If it requires an in-person fix, the machining can be paused until the person is able to get to the machine and service it. In some cases, the issue may be managed remotely as well, getting machines back online with minimal downtime.
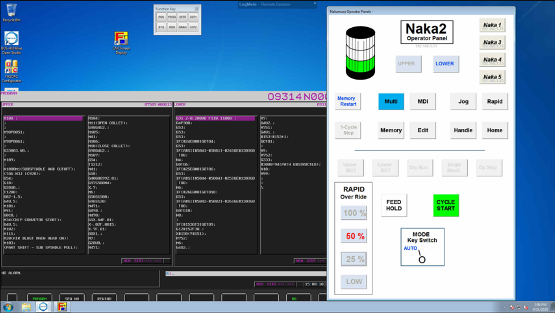
In manufacturing automation, machine control and monitoring may be accessed by an HMI on a PC or smart device. Remote access is also available, as seen here. Images courtesy of Lowell.
Another area of cost savings relates to quality. When parts are measured throughout production, it may reduce the risk of non-conforming parts that need to be remade or reworked. Without the constant monitoring automation provides, it’s possible for there to be latency between when an issue is identified during in-process inspection and then corrected. For example, if a machine makes 20 parts per hour, and it’s been running for 10 minutes before the process is fixed, there could be three non-conforming parts that need to be scrapped or re-inspected. Automation could identify the issue and quickly fix it before additional, non-conforming parts are made.
Finally, post-machining inspection time may also be reduced with automation. There is a greater level of confidence the machined parts meet requirements, and there’s supporting data in the control system. Without automation, all features need to be sample inspected as part of final inspection. If a dimension is out of tolerance at this stage, it can lead to significant sorting and rework.
Automation Contributes to Better Product Results
In the handoff of a drawing from the engineering team to the manufacturer, it becomes the manufacturer’s responsibility to create a part and features that meet design intent and conform to requirements.
While automation isn’t a process typically visible beyond the manufacturing floor, it often makes a big difference in delivering quality parts that meet expected timelines and budgets. With advantages from reducing lead time and flexing capacity to more consistent product results, manufacturers will continue to invest in automation upgrades that benefit their customers and help them remain competitive.
Jim Stertz is the vice president of automation/technology for Lowell Inc. He drives the automation, robotics, and technology efforts at Lowell, which led to breakthroughs in efficiency, quality, and spindle uptime. Stertz previously managed Lowell’s quality efforts for 35 years, including implementation of the ISO Quality Management System.
While not always visible to the customer, one way manufacturers can meet these expectations is through automated machining processes. Automation opens new ways to create a more consistent part at the point of manufacturing. It can also help manufacturers add capacity in their existing shop footprint and reduce the labor hours per part to positively deliver on lead times and cost.
This article offers a closer look at how automation may work behind the scenes to improve quality, delivery, and cost.
Automation for Quality: More Consistent Results
Automation can be applied at various points in manufacturing, and its application to machine tools can lead to quality improvements.
A component’s machining process will vary depending on the machine, tooling, material, or environment. The machinist needs to be familiar with all aspects of the process to stay ahead of any potential drift in a feature’s size or location. They also need to be able to correctly offset the machine program to ensure ongoing conformance.
The number of variables in the process can be staggering. On the simpler end, a part may require 10 tools and have 20 features and dimensions to track. Some of the most complex parts may require 50 tools and 100 features and dimensions. This quickly escalates the complexity of managing the machining process and introduces additional opportunities for error.
Automating in-machine measurement can help across the range of parts. One way this happens is through in-machine probes. These probes measure tool cuts and dimensions during production at the machine level, and the software tracks which cuts and dimensions are made by which tools. The machine program reads the measured results and software conditional statements decide to continue the process, make tool offsets, or stop the activity.
The analysis may show any number of results, depending on the programming. Three main categories of results include:
- Category 1: The measurement conforms to requirements, and production continues as is.
- Category 2: The measurement begins to drift and will not conform unless action is taken. Machine control makes an adjustment to the tool offset as production continues.
- Category 3: Something is wrong with the process, such as a tool breaking. Production stops so a machinist can fix the issue.
Automation for Delivery: Additional Capacity Extends Production Limits
Another way automation drives results for device companies is in delivery and lead times. When contract manufacturers automate machines, it can often lead to around-the-clock machining. This can increase capacity within an existing facility.
As an example, a non-automated machine may run 50 to 100 hours per week, depending on the number of orders and shifts. Typically, when machinists aren’t around, the machine isn’t running.
Since automation is monitored by the machine control, a machine’s production capacity may be extended to up to 24 hours a day. A machine previously running at 50 to 100 hours per week may now be able to run 150 to 168 hours a week. This allows the manufacturer to produce more parts each week, shortening the overall lead time.

Machine control systems are programmed to read measurement results, and can be accessed remotely as seen here. Based on results, software conditional statements decide whether to continue the process, make tool offsets, or stop the process.
Automation for Cost: Around-the-Clock Production
Labor hours are the most expensive component of machining. Automation allows fewer people to manage the machining process, reducing the labor hours required for each part. This may lead to customer savings, because what used to require multiple people can now be managed by fewer machinists. It also enables domestic manufacturers to level the playing field compared to off-shore sources, and keep in balance the rising costs of doing business.

Operator panels give machinists control over the manufacturing process as they monitor production, and are accessible remotely as seen here.
This doesn’t necessarily mean automation replaces machinist jobs. In the best scenarios, it means fewer hands have to be involved to accomplish work, and machinists are able to accomplish more complex tasks in their day. Additionally, improved quality, shorter lead times, and lower labor hours per part machined should allow a manufacturer to be more competitive, which may result in a higher volume of work for the machining team.
With automation, if something changes in the process, remote monitoring and alerts from the machine control can notify the machinist of an issue even when they aren’t in the building. If it requires an in-person fix, the machining can be paused until the person is able to get to the machine and service it. In some cases, the issue may be managed remotely as well, getting machines back online with minimal downtime.

In manufacturing automation, machine control and monitoring may be accessed by an HMI on a PC or smart device. Remote access is also available, as seen here. Images courtesy of Lowell.
Another area of cost savings relates to quality. When parts are measured throughout production, it may reduce the risk of non-conforming parts that need to be remade or reworked. Without the constant monitoring automation provides, it’s possible for there to be latency between when an issue is identified during in-process inspection and then corrected. For example, if a machine makes 20 parts per hour, and it’s been running for 10 minutes before the process is fixed, there could be three non-conforming parts that need to be scrapped or re-inspected. Automation could identify the issue and quickly fix it before additional, non-conforming parts are made.
Finally, post-machining inspection time may also be reduced with automation. There is a greater level of confidence the machined parts meet requirements, and there’s supporting data in the control system. Without automation, all features need to be sample inspected as part of final inspection. If a dimension is out of tolerance at this stage, it can lead to significant sorting and rework.
Automation Contributes to Better Product Results
In the handoff of a drawing from the engineering team to the manufacturer, it becomes the manufacturer’s responsibility to create a part and features that meet design intent and conform to requirements.
While automation isn’t a process typically visible beyond the manufacturing floor, it often makes a big difference in delivering quality parts that meet expected timelines and budgets. With advantages from reducing lead time and flexing capacity to more consistent product results, manufacturers will continue to invest in automation upgrades that benefit their customers and help them remain competitive.
Jim Stertz is the vice president of automation/technology for Lowell Inc. He drives the automation, robotics, and technology efforts at Lowell, which led to breakthroughs in efficiency, quality, and spindle uptime. Stertz previously managed Lowell’s quality efforts for 35 years, including implementation of the ISO Quality Management System.