Gregory Grissett, Principal and Chair, Intellectual Property Group, Offit Kurman P.A.09.14.21
The Monadnock region in southern New Hampshire (where I have the pleasure of living) is dominated by Mount Monadnock and tradition holds that “Monadnock” means “stands above the rest.” What does a mountainous region in New Hampshire have to do with IP strategy? Medical device companies with a thoughtful IP strategy typically have higher valuations, higher revenue, and more robust product offerings, compared to those without a thoughtful IP strategy. What does it take to develop and execute an effective patent strategy that stands above the rest? Part I described components of a robust patent strategy in the medical device sector. This part explains how to mitigate risk related to third-party patent rights.
Foundational Laws Related to Patent Risk Mitigation and Freedom-to-Operate
U.S. patent law establishes the broad right of a patent owner to prevent others from making, using, selling, offering for sale, or importing into the United States, a product or service that is claimed in a valid patent.1 When another company performs any of these infringing acts without permission from the patent owner, the patent owner is entitled to money damages and injunctive relief (i.e., the ability to bar a third party from selling a product in the U.S. covered by a patent).2
Liability for direct patent infringement attaches to a company when 1) the patent owner proves a company’s product infringes a patent, and 2) the defendant-company fails to establish3 the patent claim is invalid or unenforceable. In addition, patent infringement liability can also attach to a company that induces a third party to infringe a patent claim. Inducement liability is established when the alleged infringer A) engages in conduct that induces or encourages a third party to infringe the patent, and B) had knowledge that the induced acts comprise patent infringement.4 For example, a medical device company can induce a doctor to infringe a method claim of a patent and be liable for patent infringement even though that company did not perform the infringing acts itself.
Willful infringement is another risk. When willful infringement is established, a court may triple the damage award and/or add attorney’s fees.
Finally, patent liability attaches only to companies found to infringe valid patent claims. Failure of a patent claim to comply with patentability principles renders that claim invalid and ineffective to prohibit infringers from selling a competing product.5
U.S. patent litigation is complex and costly. The threat of money damages, injunctions, and defense costs are considerable incentives to design products that are unique and do not infringe the other’s patent rights.
Why Should You Implement a Freedom-to-Operate Strategy?
A freedom-to-operate (FTO) study can help a medical device company mitigate patent infringement risk by identifying and evaluating the extent and nature of patent risks associated with selling your product. FTO studies also guide the design and development of your product by directing a development toward solutions with lower risk. In addition, FTO studies give confidence to investors that their investment is not an investment in a lawsuit. Furthermore, formal patent opinions of counsel, which are often prepared during an FTO study, may be used as evidence to disprove both willful infringement and inducement infringement allegations.
How Do You Conduct an FTO Analysis?
FTO studies have four phases: 1) patent search or notification of patent rights, 2) infringement analysis, 3) design around activities, and 4) validity analysis (Figure 1).
Identifying patents that are relevant to your company’s products is the first phase in an FTO study. This can include engaging a patent search firm to locate patents and published applications relevant to the product. Your company may receive a cease and desist letter asserting your company’s product infringes a patent. In any event, once potentially relevant patents are identified, preliminary analysis of the results can filter out irrelevant and expired patents.
In phase 2, patent counsel analyzes all the patents found in phase 1. In phase 2, the claims, the specification, the patent file history, and relevant claim terms are construed.6 Then, the construed claims are compared to the product. If all the claim elements are found in the product, then the product infringes the claim. If any part of the claim is not present in the product, there is no infringement.
For example, as shown in Figure 2, product A includes all the elements required in claim 1. Product B, however, does not have all required in claim 1—no grey gizmo—and therefore, does not literally infringe the claim. While this small difference may avoid literal infringement, a risk may still present because it is conceivable a court may find product B infringes the claim under Doctrine of Equivalents. Subtle details in this case can make all the difference. Phase 2 is where engaged patent counsel can help identify the contours of the patent rights so the product development team can understand how to proceed in development.
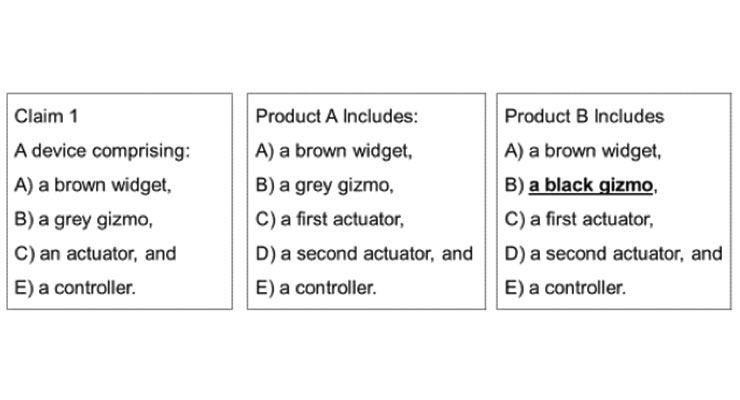
Figure 2
If the product does not literally or equivalently infringe the claim, there is low patent risk (green box in Figure 1) and the product has freedom-to-operate. If, however, the product at issue infringes the patent claim, design changes are considered in phase 3.
Phase 3 is classic design around analysis. If the product can be modified to avoid the claim, infringement risk is minimized and freedom-to-operate is reasonably attained. Timing is important. If a PMA or 510(k) application has been filed, it may be very costly and time consuming to change the product. In those circumstances, business needs may require progression to phase 4: validity analysis.
In phase 4, patent validity is analyzed. This may involve conducting a validity patent search to locate invalidating prior art. The results of the search can be used to prepare an opinion of counsel or be used as basis to invalidate the claims in an inter partes review or ex parte reexamination at the U.S. Patent & Trademark Office. Other grounds for invalidity, such as violation of the written description requirement or the claims are not properly enabled, can be considered. If there is a high probability the patent claim is invalid, there is low patent risk and the product has freedom-to-operate. Sometimes, the patent claim is not as susceptible to an invalidity attack. In that case, one can secure a license, purchase the patent, or proceed in the face of the risk.
FTO studies result in practical business tools. First, the materials prepared during an FTO study can be used as a basis to address concerns an investor or strategic partner may have in view of the patent risks identified in phases 1-3. Second, companies that proactively conduct an FTO study are better positioned to address and manage due diligence. Third, FTO results may inform how important contracts are drafted as they relate to IP risk. Fourth, written patent opinions may be used as evidence to disprove a finding of willful infringement or inducement infringement of a patent claim. Finally, FTO studies can guide development toward wholly unique products and services that can result in independent patent rights.
When Should You Conduct Patent Risk Mitigation Analysis?
An FTO study should be conducted when driven by key commercial milestones or external events. For commercial milestones, timing is (almost) everything. In general, the earlier in the product development phase you can conduct an FTO study, the better. Early in the design and development phase, before design freeze occurs, you have the flexibility to make changes to the product in the face of patent risks uncovered by the FTO study. This allows you to make changes that are not (as) costly. In the medical device context, FTO studies should ideally occur before 1) significant capital outlay, 2) filing a 510(k) or PMA, and 3) commercial sales occur. The more finalized the product design, the more robust the FTO results will be.
External events that require FTO studies may be investor due diligence or receipt of notice/cease & desist letter. Potential investors and strategic partners may require it prior to making their financial placements. In the case of notice/C&D letter, timely investigation and assessment of the claims in view of the product are critical. The key issue is to address this risk proactively and ahead of instances that are critical for business or capital development.
Conclusion
Patent infringement risk is significant in the medical device sector. Infringing a valid patent can result in significant damage awards and an injunction. The threat of money damages, injunctions, and defense costs are considerable incentives to design wholly unique products that do not infringe the rights of others. A proactive FTO study can help assess and manage this risk. Timing is important and earlier assessments allow your business to pivot to avoid and manage known risks.
References
Foundational Laws Related to Patent Risk Mitigation and Freedom-to-Operate
U.S. patent law establishes the broad right of a patent owner to prevent others from making, using, selling, offering for sale, or importing into the United States, a product or service that is claimed in a valid patent.1 When another company performs any of these infringing acts without permission from the patent owner, the patent owner is entitled to money damages and injunctive relief (i.e., the ability to bar a third party from selling a product in the U.S. covered by a patent).2
Liability for direct patent infringement attaches to a company when 1) the patent owner proves a company’s product infringes a patent, and 2) the defendant-company fails to establish3 the patent claim is invalid or unenforceable. In addition, patent infringement liability can also attach to a company that induces a third party to infringe a patent claim. Inducement liability is established when the alleged infringer A) engages in conduct that induces or encourages a third party to infringe the patent, and B) had knowledge that the induced acts comprise patent infringement.4 For example, a medical device company can induce a doctor to infringe a method claim of a patent and be liable for patent infringement even though that company did not perform the infringing acts itself.
Willful infringement is another risk. When willful infringement is established, a court may triple the damage award and/or add attorney’s fees.
Finally, patent liability attaches only to companies found to infringe valid patent claims. Failure of a patent claim to comply with patentability principles renders that claim invalid and ineffective to prohibit infringers from selling a competing product.5
U.S. patent litigation is complex and costly. The threat of money damages, injunctions, and defense costs are considerable incentives to design products that are unique and do not infringe the other’s patent rights.
Why Should You Implement a Freedom-to-Operate Strategy?
A freedom-to-operate (FTO) study can help a medical device company mitigate patent infringement risk by identifying and evaluating the extent and nature of patent risks associated with selling your product. FTO studies also guide the design and development of your product by directing a development toward solutions with lower risk. In addition, FTO studies give confidence to investors that their investment is not an investment in a lawsuit. Furthermore, formal patent opinions of counsel, which are often prepared during an FTO study, may be used as evidence to disprove both willful infringement and inducement infringement allegations.
How Do You Conduct an FTO Analysis?
FTO studies have four phases: 1) patent search or notification of patent rights, 2) infringement analysis, 3) design around activities, and 4) validity analysis (Figure 1).
Identifying patents that are relevant to your company’s products is the first phase in an FTO study. This can include engaging a patent search firm to locate patents and published applications relevant to the product. Your company may receive a cease and desist letter asserting your company’s product infringes a patent. In any event, once potentially relevant patents are identified, preliminary analysis of the results can filter out irrelevant and expired patents.
In phase 2, patent counsel analyzes all the patents found in phase 1. In phase 2, the claims, the specification, the patent file history, and relevant claim terms are construed.6 Then, the construed claims are compared to the product. If all the claim elements are found in the product, then the product infringes the claim. If any part of the claim is not present in the product, there is no infringement.
For example, as shown in Figure 2, product A includes all the elements required in claim 1. Product B, however, does not have all required in claim 1—no grey gizmo—and therefore, does not literally infringe the claim. While this small difference may avoid literal infringement, a risk may still present because it is conceivable a court may find product B infringes the claim under Doctrine of Equivalents. Subtle details in this case can make all the difference. Phase 2 is where engaged patent counsel can help identify the contours of the patent rights so the product development team can understand how to proceed in development.

Figure 2
If the product does not literally or equivalently infringe the claim, there is low patent risk (green box in Figure 1) and the product has freedom-to-operate. If, however, the product at issue infringes the patent claim, design changes are considered in phase 3.
Phase 3 is classic design around analysis. If the product can be modified to avoid the claim, infringement risk is minimized and freedom-to-operate is reasonably attained. Timing is important. If a PMA or 510(k) application has been filed, it may be very costly and time consuming to change the product. In those circumstances, business needs may require progression to phase 4: validity analysis.
In phase 4, patent validity is analyzed. This may involve conducting a validity patent search to locate invalidating prior art. The results of the search can be used to prepare an opinion of counsel or be used as basis to invalidate the claims in an inter partes review or ex parte reexamination at the U.S. Patent & Trademark Office. Other grounds for invalidity, such as violation of the written description requirement or the claims are not properly enabled, can be considered. If there is a high probability the patent claim is invalid, there is low patent risk and the product has freedom-to-operate. Sometimes, the patent claim is not as susceptible to an invalidity attack. In that case, one can secure a license, purchase the patent, or proceed in the face of the risk.
FTO studies result in practical business tools. First, the materials prepared during an FTO study can be used as a basis to address concerns an investor or strategic partner may have in view of the patent risks identified in phases 1-3. Second, companies that proactively conduct an FTO study are better positioned to address and manage due diligence. Third, FTO results may inform how important contracts are drafted as they relate to IP risk. Fourth, written patent opinions may be used as evidence to disprove a finding of willful infringement or inducement infringement of a patent claim. Finally, FTO studies can guide development toward wholly unique products and services that can result in independent patent rights.
When Should You Conduct Patent Risk Mitigation Analysis?
An FTO study should be conducted when driven by key commercial milestones or external events. For commercial milestones, timing is (almost) everything. In general, the earlier in the product development phase you can conduct an FTO study, the better. Early in the design and development phase, before design freeze occurs, you have the flexibility to make changes to the product in the face of patent risks uncovered by the FTO study. This allows you to make changes that are not (as) costly. In the medical device context, FTO studies should ideally occur before 1) significant capital outlay, 2) filing a 510(k) or PMA, and 3) commercial sales occur. The more finalized the product design, the more robust the FTO results will be.
External events that require FTO studies may be investor due diligence or receipt of notice/cease & desist letter. Potential investors and strategic partners may require it prior to making their financial placements. In the case of notice/C&D letter, timely investigation and assessment of the claims in view of the product are critical. The key issue is to address this risk proactively and ahead of instances that are critical for business or capital development.
Conclusion
Patent infringement risk is significant in the medical device sector. Infringing a valid patent can result in significant damage awards and an injunction. The threat of money damages, injunctions, and defense costs are considerable incentives to design wholly unique products that do not infringe the rights of others. A proactive FTO study can help assess and manage this risk. Timing is important and earlier assessments allow your business to pivot to avoid and manage known risks.
References
- 35 U.S.C. §271
- 35 U.S.C. §284
- 35 U.S.C. §302-329
- 35 U.S.C. §271(b), Global-Tech Appliances, Inc. v. SEB S.A., 563 U.S. 754 (2011)
- 35 U.S.C. §101, §102 and § 103
- Claim construction is a fundamental and required step in evaluating any patent risk.













