Gerald McDonnell, BSc, Ph.D. , Senior Director, Microbiological Quality & Sterility Assurance, J&J09.16.22
Orthopedic implant procedures are among the most successful surgeries in the last century. These life improving operations have low levels of complications, particularly with the application of best practices in reducing risks.1,2 Despite these low rates, with increasing numbers of surgical procedures to implant devices daily, the incidence of complications (including infections) will continue to be a risk despite aseptic protocols. Failure in the surgical implantation of a device can be devastating, especially when it is due to surgical site infections.
It is important to consider a higher percentage of infections are linked to endogenous sources of microorganisms from patients, but a lower percentage are from exogenous (e.g., environmental) sources. In addition, there is a concern with the increased patient risks with microorganisms tolerant or resistant to antibiotic therapy. Persistence of microorganisms on device surfaces or in damaged tissue can lead to biofilm development over time, with significant patient impact including premature revision surgery or subsequent life altering events.3
This article expounds on the outcomes of recent U.S. Food and Drug Administration (FDA) workshops calling for new ideas and regulatory innovations to reduce or remove these relatively low occurring but highly devastating infections.4
Implant-associated infections involve interactions between the pathogen, the implant, and the patient’s immune system. Studies confirm that with or without a foreign body (implant), tissue contamination by opportunistic pathogens are often successfully resolved by the host immune response, but the presence of a foreign body introduced during surgery can lead to microbial persistence, formation of granulation tissue overtime, and subsequent fibrous encapsulation. This generates an immune toleration that predisposes an implant to microbial persistence and infection.6 The basis for this is the ability of vegetative microorganisms (particularly bacteria) to attach to and multiply on the surface with the subsequent development of a biofilm structure over time (Figure 1). Once formed, biofilms are notoriously persistent and difficult to treat with traditional antibiotic therapy, with resolution often requiring implant removal and revision. Therefore, the greatest impact in reducing the risk of biofilm development and serious infection is at the source of attachment and initial multiplication.
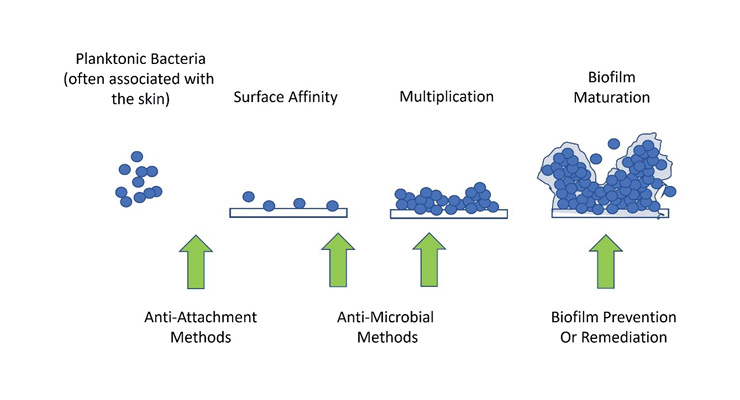
Figure 1: Biofilm development and infection. Note that prevention of adhesion/attachment and colonization have the greatest opportunity to reduce the risk of orthopedic implant-associated infections.
An additional challenge is that joint replacement implants are designed to integrate with bone and often include substrate materials or coatings that promote cellular adhesion or integration into anatomic structures. These very same surface properties are often susceptible to bacterial attachment during surgical implantation and potential biofilm formation. Overall, despite best practices in wound and surgical management, it remains possible for microorganisms to be introduced into the implant/tissue area and present a risk of infection development.
Deep implant-associated infections traditionally develop within three months after surgery and are classified as early postoperative infections. Delayed or subacute infections develop three to 24 months after surgery, and late infections can be detected after more than 24 months. Recent classifications further distinguish early infections as manifesting within one month after surgery, acute hematogenous infections with a duration of symptoms up to three weeks, and chronic infections that persist for more than three weeks.7 These can require different treatment regimens, but all or most can be prevented at the point of surgical implantation. Infection rates with different microorganisms can vary depending on the geographic areas.8 In general, skin-based microorganisms (e.g., staphylococci) are the most frequently isolated pathogens associated with total arthroplasty, with S. epidermidis being the most reported in the U.S. and S. aureus more common in Europe (Table 1).9-11 The major sources of these bacteria are considered to be from the patient skin/wound, the operating room environment, and the handling/use of devices or other materials used during surgery. Given best practices in operating room management, the levels of microbial contamination are considered very low with typical estimates <1-100 CFU/device in the environment or associated device used during surgery.12,13 Reducing this risk at the point of implantation is, therefore, important to minimize infection development.
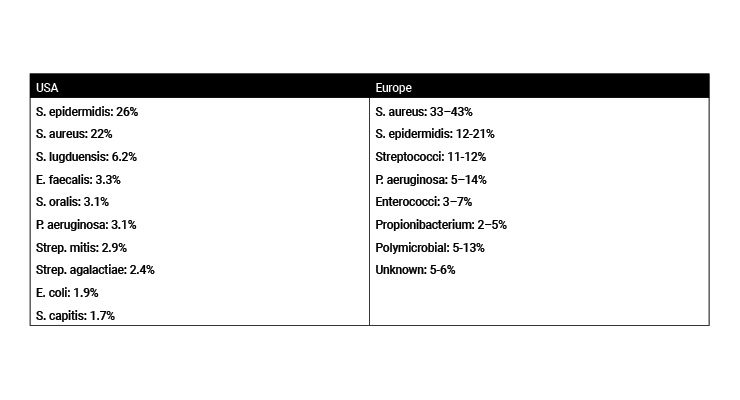
Table 1: Most frequently isolated pathogens associated with total arthroplasty infections.10,11
Antibiotics can also play a critical role in reducing risks of surgical site infections. But there are concerns on the reduced impact of antibiotic effectiveness given the growing rates of antibiotic resistance in bacteria. Risks not only include gram-positive bacteria, but also increasing reports of infections with gram-negative bacteria with broad-spectrum resistance profiles.14 Prudent and controlled use of anti-infectives is, therefore, important. Prophylactic, short-term antibiotic administration is critical to reducing surgical site infections,1,2 along with best surgical aseptic practices in the prevention of infection from endogenous or exogenous sources. Antibiotics and/or microbicides can be used directly as preventative measures during or following the implant placement. This can be achieved by either incorporating antimicrobials directly into implant surface designs or by general application of antimicrobials during surgery to the device/wound at the time of surgery. It is preferable to use the former approach to improve clinical outcomes and reduce the overall concentrations of antibiotics used.
Independent of the technology being considered, a risk assessment should first consider a review of the literature and established information on the technology being used. As an example, for anti-infectives including antibiotics and microbicides, this should include what is already known about the spectrum of efficacy, risks of resistance, and toxicological risks at the desired concentrations to be used over time. Only in the case of new or novel anti-infective technologies would verification studies be necessary, to include minimum inhibitory concentration (MIC) and/or minimum bactericidal concentration (MBC) studies, on the types of microorganisms commonly associated with surgical site (specifically orthopedic) infections. Equally, in many of these cases, the toxicological risks associated with the use of established technologies (such as antibiotics) is well understood and extensive additional studies should only be necessary with new anti-infectives.
Devices or technologies can then be shown to be effective against specific microorganisms from in-vitro, cadaveric, or animal testing that demonstrate defined reductions in low levels of viable microorganisms or reduced potential of microbial attachment without detriment to the device or the subject. We should encourage the development of practical in-vitro test methods to quantitatively demonstrate the efficacy of antimicrobial and anti-adhesion technologies under conditions that simulate the contamination levels during surgical implantation. A separate series of test methods and associated claims can be similarly developed for use in treating known cases of developing or advanced infection cases, typified by the development of a stable biofilm.
These test methods should be acceptable to allow for regulatory clearance of new claim structures without needing full clinical trials or overall infection rate reduction demonstration. As a demonstration of effectiveness, the traditional standard is the use of randomized clinical trial (RCT) showing improved effects on infection rates for a claim associated with infection reduction. Due to the overall low percentage of infection rates with orthopedic implant replacement surgery (reported in sources as low as <1% to approximately 3% in certain patient populations), demonstrating benefit (decrease in the rate of infection) involves prohibitively large populations of patients to conclude meaningful results. Large sample sizes and long-term commitments are costly clinical studies that lack incentives for the orthopedic industry to bring new technologies to the market in the U.S.15 Real-world evidence could continue to show the overall benefits of these preventative technologies overtime.
To achieve this goal, it is recommended manufacturers and regulators should utilize:
References
Dr. Gerald McDonnell is a senior director for Microbiological Quality & Sterility Assurance at Johnson & Johnson. He serves as the global technical leader in microbiology and contamination control including cleaning, disinfection, sterilization, device processing and microbiology. Prior to J&J, he was a vice president of Scientific and Clinical Services for STERIS Corporation in Mentor, Ohio. He has published over 180 publications and patents and is a member of national and international working groups for standard and guideline development. Dr. McDonnell can be reached at 908-927-2865 or gmcdonne@its.jnj.com.
The Orthopaedic Surgical Manufacturers Association (OSMA; https://osma.net/) is a nonprofit organization whose membership consists of manufacturers of orthopedic surgical appliances, implants, instruments or equipment and orthobiologics. Since its inception in 1954, OSMA has continued to actively participate in standards and regulatory guideline development, educate membership on regulatory matters and provide regulatory professionals a forum to collaborate, communicate, cooperate, and interact with worldwide regulatory agencies and healthcare professionals. The OSMA Anti-Infective Working Group include G. McDonnell, R. Durgin, R. Harten, S. Bonnell, S. Datta, J. Meisner, D. Hickey, J. Rose, T. Fearnley, J. Dedania, D. Joiner-Fox, K. Shah, R. Cantrell, C. Engleman, J. Zak, L. Cousin.
It is important to consider a higher percentage of infections are linked to endogenous sources of microorganisms from patients, but a lower percentage are from exogenous (e.g., environmental) sources. In addition, there is a concern with the increased patient risks with microorganisms tolerant or resistant to antibiotic therapy. Persistence of microorganisms on device surfaces or in damaged tissue can lead to biofilm development over time, with significant patient impact including premature revision surgery or subsequent life altering events.3
This article expounds on the outcomes of recent U.S. Food and Drug Administration (FDA) workshops calling for new ideas and regulatory innovations to reduce or remove these relatively low occurring but highly devastating infections.4
Sources of Infection
There are many common ways microorganisms, particularly bacteria, can enter the body, such as directly through wounds (breaks or cuts in the skin), during dental procedures (e.g., tooth extractions or root canal procedures), environmental or skin cross-contamination of surgical devices used during procedures, operating room air quality, and inefficient patient skin disinfection (antiseptic) procedures.5 Individual patient risk profiles vary widely with some having higher risks of infections due to underlying conditions such as immune deficiencies, diabetes, poor circulation, chemotherapy or corticosteroid treatments, and obesity.1,2 Reducing the risk of infection requires thorough patient health management, prudent antibiotic use, and strict aseptic technique in preparation for and during surgery. But even with these best practices, microbiological contamination from the environment and patient wound, albeit often being low, can still account for devastating consequences to a small portion of patients. There is a particular opportunity to reduce the risk of such infections at the initial source of microbial attachment or growth pertaining to implant surgery.Implant-associated infections involve interactions between the pathogen, the implant, and the patient’s immune system. Studies confirm that with or without a foreign body (implant), tissue contamination by opportunistic pathogens are often successfully resolved by the host immune response, but the presence of a foreign body introduced during surgery can lead to microbial persistence, formation of granulation tissue overtime, and subsequent fibrous encapsulation. This generates an immune toleration that predisposes an implant to microbial persistence and infection.6 The basis for this is the ability of vegetative microorganisms (particularly bacteria) to attach to and multiply on the surface with the subsequent development of a biofilm structure over time (Figure 1). Once formed, biofilms are notoriously persistent and difficult to treat with traditional antibiotic therapy, with resolution often requiring implant removal and revision. Therefore, the greatest impact in reducing the risk of biofilm development and serious infection is at the source of attachment and initial multiplication.

Figure 1: Biofilm development and infection. Note that prevention of adhesion/attachment and colonization have the greatest opportunity to reduce the risk of orthopedic implant-associated infections.
An additional challenge is that joint replacement implants are designed to integrate with bone and often include substrate materials or coatings that promote cellular adhesion or integration into anatomic structures. These very same surface properties are often susceptible to bacterial attachment during surgical implantation and potential biofilm formation. Overall, despite best practices in wound and surgical management, it remains possible for microorganisms to be introduced into the implant/tissue area and present a risk of infection development.
Deep implant-associated infections traditionally develop within three months after surgery and are classified as early postoperative infections. Delayed or subacute infections develop three to 24 months after surgery, and late infections can be detected after more than 24 months. Recent classifications further distinguish early infections as manifesting within one month after surgery, acute hematogenous infections with a duration of symptoms up to three weeks, and chronic infections that persist for more than three weeks.7 These can require different treatment regimens, but all or most can be prevented at the point of surgical implantation. Infection rates with different microorganisms can vary depending on the geographic areas.8 In general, skin-based microorganisms (e.g., staphylococci) are the most frequently isolated pathogens associated with total arthroplasty, with S. epidermidis being the most reported in the U.S. and S. aureus more common in Europe (Table 1).9-11 The major sources of these bacteria are considered to be from the patient skin/wound, the operating room environment, and the handling/use of devices or other materials used during surgery. Given best practices in operating room management, the levels of microbial contamination are considered very low with typical estimates <1-100 CFU/device in the environment or associated device used during surgery.12,13 Reducing this risk at the point of implantation is, therefore, important to minimize infection development.

Table 1: Most frequently isolated pathogens associated with total arthroplasty infections.10,11
Antibiotics can also play a critical role in reducing risks of surgical site infections. But there are concerns on the reduced impact of antibiotic effectiveness given the growing rates of antibiotic resistance in bacteria. Risks not only include gram-positive bacteria, but also increasing reports of infections with gram-negative bacteria with broad-spectrum resistance profiles.14 Prudent and controlled use of anti-infectives is, therefore, important. Prophylactic, short-term antibiotic administration is critical to reducing surgical site infections,1,2 along with best surgical aseptic practices in the prevention of infection from endogenous or exogenous sources. Antibiotics and/or microbicides can be used directly as preventative measures during or following the implant placement. This can be achieved by either incorporating antimicrobials directly into implant surface designs or by general application of antimicrobials during surgery to the device/wound at the time of surgery. It is preferable to use the former approach to improve clinical outcomes and reduce the overall concentrations of antibiotics used.
Design Strategies to Reduce the Risk
The uses of antimicrobial and other technologies have been described that can reduce the attachment and multiplication of microorganisms on implant surfaces and can show a clinical benefit in reducing the attachment of low levels of bacteria as the first step in the development of an infection and/or biofilm development. Some of these technologies are already used in surgical practice internationally, but are often not cleared for use in the U.S. These anti-infective or anti-attachment surface technologies can have the greatest opportunity to reduce infection rates at the point of implantation. Alternative and practical regulatory expectations can help in the successful use of these technologies. It is recommended to consider a risk-benefit approach in the consideration of these technologies, the development of practical test methods, and associated regulatory pathways for clearance of a new classification of implants that can be shown to reduce or prevent the attached and/or growth of microorganisms.Independent of the technology being considered, a risk assessment should first consider a review of the literature and established information on the technology being used. As an example, for anti-infectives including antibiotics and microbicides, this should include what is already known about the spectrum of efficacy, risks of resistance, and toxicological risks at the desired concentrations to be used over time. Only in the case of new or novel anti-infective technologies would verification studies be necessary, to include minimum inhibitory concentration (MIC) and/or minimum bactericidal concentration (MBC) studies, on the types of microorganisms commonly associated with surgical site (specifically orthopedic) infections. Equally, in many of these cases, the toxicological risks associated with the use of established technologies (such as antibiotics) is well understood and extensive additional studies should only be necessary with new anti-infectives.
Devices or technologies can then be shown to be effective against specific microorganisms from in-vitro, cadaveric, or animal testing that demonstrate defined reductions in low levels of viable microorganisms or reduced potential of microbial attachment without detriment to the device or the subject. We should encourage the development of practical in-vitro test methods to quantitatively demonstrate the efficacy of antimicrobial and anti-adhesion technologies under conditions that simulate the contamination levels during surgical implantation. A separate series of test methods and associated claims can be similarly developed for use in treating known cases of developing or advanced infection cases, typified by the development of a stable biofilm.
These test methods should be acceptable to allow for regulatory clearance of new claim structures without needing full clinical trials or overall infection rate reduction demonstration. As a demonstration of effectiveness, the traditional standard is the use of randomized clinical trial (RCT) showing improved effects on infection rates for a claim associated with infection reduction. Due to the overall low percentage of infection rates with orthopedic implant replacement surgery (reported in sources as low as <1% to approximately 3% in certain patient populations), demonstrating benefit (decrease in the rate of infection) involves prohibitively large populations of patients to conclude meaningful results. Large sample sizes and long-term commitments are costly clinical studies that lack incentives for the orthopedic industry to bring new technologies to the market in the U.S.15 Real-world evidence could continue to show the overall benefits of these preventative technologies overtime.
Perspectives for the Future
Given the devastating consequences on the patients affected, we should look to supply a practical rationale to improve the anti-infective nature of orthopedic implants and improved patient outcomes. Most devastating or catastrophic outcomes of joint infections are within a very small category of patients, and these infections are often multi-factorial. There is an important road forward to allow for the development and approval processes for device technologies to claim effectiveness in reducing the risks of infection with the microorganisms known to be of the most concern for implant infections. It should also be understood that potential benefits without specific, significant harm to most patients would be beneficial to individuals susceptible to or at a higher risk of infection.To achieve this goal, it is recommended manufacturers and regulators should utilize:
- A risk-assessment-based approach for evaluating antimicrobial or anti-attachment technologies for orthopedic devices. This should include evaluation of any existing safety and efficacy data on the technology.
- In-vitro test method development to quantitatively demonstrate technology efficacy. Test methods will include methods to demonstrate the quantitative reduction of microbial attachment or growth on a surface under conditions that simulate the surgical risk, and separate methods for evaluating the impact of technologies under advanced infection cases that are typified by the presence of a biofilm. Claims associated with the successful demonstration of efficacy in-vitro should be established in these cases.
- The establishment of practical approaches for the in vivo verification of efficacy and safety, without the requirement for randomized clinical trials.
References
- bit.ly/odt220921
- bit.ly/odt220922
- bit.ly/odt220923
- bit.ly/odt220924
- bit.ly/odt220925
- go.nature.com/3Ro2jv3
- bit.ly/odt220927
- amzn.to/3AylTxO
- bit.ly/odt220929
- Joachim J. Multiplexed Laboratory Test of Synovial Fluid for the Detection of Periprosthetic Joint Infection. 5th Stevens Conference on Bacteria-Materials Interactions, June 12-13, 2019.
- bit.ly/odt220931
- bit.ly/odt220932
- bit.ly/odt220933
- bit.ly/odt220934
- bit.ly/odt220935
Dr. Gerald McDonnell is a senior director for Microbiological Quality & Sterility Assurance at Johnson & Johnson. He serves as the global technical leader in microbiology and contamination control including cleaning, disinfection, sterilization, device processing and microbiology. Prior to J&J, he was a vice president of Scientific and Clinical Services for STERIS Corporation in Mentor, Ohio. He has published over 180 publications and patents and is a member of national and international working groups for standard and guideline development. Dr. McDonnell can be reached at 908-927-2865 or gmcdonne@its.jnj.com.
The Orthopaedic Surgical Manufacturers Association (OSMA; https://osma.net/) is a nonprofit organization whose membership consists of manufacturers of orthopedic surgical appliances, implants, instruments or equipment and orthobiologics. Since its inception in 1954, OSMA has continued to actively participate in standards and regulatory guideline development, educate membership on regulatory matters and provide regulatory professionals a forum to collaborate, communicate, cooperate, and interact with worldwide regulatory agencies and healthcare professionals. The OSMA Anti-Infective Working Group include G. McDonnell, R. Durgin, R. Harten, S. Bonnell, S. Datta, J. Meisner, D. Hickey, J. Rose, T. Fearnley, J. Dedania, D. Joiner-Fox, K. Shah, R. Cantrell, C. Engleman, J. Zak, L. Cousin.