Nanovis02.17.17
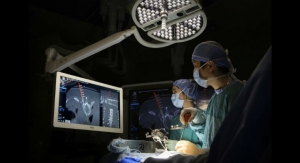
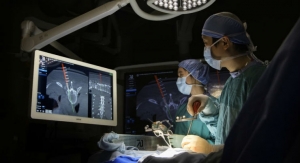
Nanovis, a life sciences company developing implant systems that reduce fixation related complications and infections, has announced the successful alpha launch of its FortiCore PLIF featuring a deeply porous titanium scaffold interdigitated with a PEEK core, and implantation of the 2,000th FortiCore implant.
“My patient’s short term response to the FortiCore PLIF has been positive. I implanted the interbody using a minimally invasive technique with no problems. The shape of the implant is favorable for multiple approach options. I’m encouraged by the deep porosity of the titanium scaffold in contact with the vertebral endplate and the pre-clinical data on this technology,” said Dr. Richard B. Rodgers of Goodman Campbell Brain and Spine (Indianapolis, Ind.), a participant in the alpha launch of the FortiCore PLIF.
With more than 2,000 FortiCore implants now used in surgery, long term clinical experience with FortiCore’s deeply porous technologies has been positive, according to the company. “In the 18 months since I started using FortiCore my patients have been doing well. Initially, I was interested in trying to improve my patient’s outcomes by using a deeply porous titanium scaffold. I’ve been pleased with the excellent radiologic on growth seen on X-ray at 8-12 weeks and the ease of imaging bone through the PEEK core,” said Dr. Gregory Hoffman of Orthopedics North East (Fort Wayne, Ind.), an early adopter of the FortiCore technology.
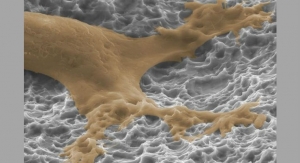
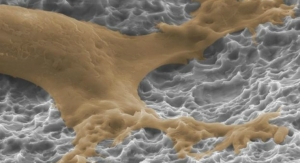
FortiCore TLIF and PLIF implants were designed with deeply porous titanium scaffolds to contact the endplate and a radiolucent, favorable-modulus PEEK (polyether ether ketone) core injection molded into the bottom of the scaffold creating a durable construct. The FortiCore TLIF and PLIF systems are available with minimally invasive surgery or open instrumentation.
“Data comparing the osseointegration strength of the FortiCore scaffold, PEEK and allograft, to the strength of trabecular host bone published in Spine in October 2016 was encouraging. One of Nanovis’ long term clinical and scientific goals is to reduce fixation related complications so we are delighted to hear positive reports from our customers about their experiences with FortiCore,” said Matt Hedrick, CEO of Nanovis.
Nanovis’ mission is to lead select markets with science-enhanced, life-improving technologies. The company’s patented and proprietary regenerative technology platforms provide differentiated surface advantages enabling the potential for existing medical devices to achieve new outcomes. Focused on aggressive, sustainable growth across multiple markets, Nanovis is commercializing science-driven platforms: the deeply porous scaffold currently available with the FortiCore line of interbody fusion devices; a developmental nanosurface technology; and developmental technology with anti-colonization and anti-microbial capabilities.
“My patient’s short term response to the FortiCore PLIF has been positive. I implanted the interbody using a minimally invasive technique with no problems. The shape of the implant is favorable for multiple approach options. I’m encouraged by the deep porosity of the titanium scaffold in contact with the vertebral endplate and the pre-clinical data on this technology,” said Dr. Richard B. Rodgers of Goodman Campbell Brain and Spine (Indianapolis, Ind.), a participant in the alpha launch of the FortiCore PLIF.
With more than 2,000 FortiCore implants now used in surgery, long term clinical experience with FortiCore’s deeply porous technologies has been positive, according to the company. “In the 18 months since I started using FortiCore my patients have been doing well. Initially, I was interested in trying to improve my patient’s outcomes by using a deeply porous titanium scaffold. I’ve been pleased with the excellent radiologic on growth seen on X-ray at 8-12 weeks and the ease of imaging bone through the PEEK core,” said Dr. Gregory Hoffman of Orthopedics North East (Fort Wayne, Ind.), an early adopter of the FortiCore technology.
FortiCore TLIF and PLIF implants were designed with deeply porous titanium scaffolds to contact the endplate and a radiolucent, favorable-modulus PEEK (polyether ether ketone) core injection molded into the bottom of the scaffold creating a durable construct. The FortiCore TLIF and PLIF systems are available with minimally invasive surgery or open instrumentation.
“Data comparing the osseointegration strength of the FortiCore scaffold, PEEK and allograft, to the strength of trabecular host bone published in Spine in October 2016 was encouraging. One of Nanovis’ long term clinical and scientific goals is to reduce fixation related complications so we are delighted to hear positive reports from our customers about their experiences with FortiCore,” said Matt Hedrick, CEO of Nanovis.
Nanovis’ mission is to lead select markets with science-enhanced, life-improving technologies. The company’s patented and proprietary regenerative technology platforms provide differentiated surface advantages enabling the potential for existing medical devices to achieve new outcomes. Focused on aggressive, sustainable growth across multiple markets, Nanovis is commercializing science-driven platforms: the deeply porous scaffold currently available with the FortiCore line of interbody fusion devices; a developmental nanosurface technology; and developmental technology with anti-colonization and anti-microbial capabilities.