When it comes to the orthopedic industry, there are a number of segments that have seen little change in terms of the technology used or advancements made. Part of this is the need to avoid altering procedures such that time is added or a change in technique is required. Surgeons will likely reject any device advancement if it does either of these.
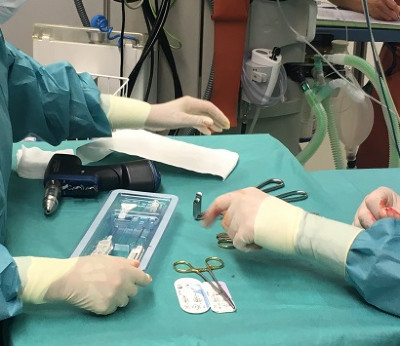
The move to ASCs is accelerated for ortho and spine procedures with the adoption of sterile-pack and surgery-ready instrument sets that eliminate reprocessing and promote faster OR turnover. ECA Medical is the single-use arm to implant OEMs developing optimized and tailored surgical solutions for a wide range of procedures, including trauma, extremities, spine, and robot-assisted.
To address the impact of single-use instruments as well as the company’s sterile-pack offerings, Lane Hale, president and CEO of ECA Medical, responded to several questions on these topics. In the following Q&A, he outlines the benefits of these technologies, how they improve upon more traditional alternatives, and how they fit with other changing dynamics within the industry, such as the shift of procedures to ambulatory surgery center (ASC).
Sean Fenske: As a supplier of orthopedic device technologies, what trends are you seeing in the industry that shape the types of products you develop and support?
Lane Hale: We’re seeing incremental updates to products, investments in digital solutions and robotics, committed movement of surgeries to the ASC, and general cost cutting. We’re also seeing more outsourcing of product development and the need for turnkey solutions by implant OEMs who are focused on sales and customer engagement, for example, upselling and cross selling a broad range of products and services as part of the continuum of care and to establish barriers to competition. Lastly, we are seeing a movement by implant OEMs to single-use instrumentation and sterile pack, surgery ready procedural kits. This shift is aimed at reducing cost, improving overall efficiency, increasing scalability in sales, streamlining logistics support, and accelerating operating room turnover at both hospitals and ASCs.

ECA’s precision TruTORQ™ single-use torque limiters are factory set, providing perfect implant fixation. High-torque spine torque limiters range from 6.0 Nm to 14 Nm and operate with tight tolerance for a full scoliosis case.
Hale: They solve many pain points felt across the industry today, including cost of owning and supporting reusable sets and instruments such as torque limiters that require reprocessing and recalibration. Single-use instruments and procedural kits also address concerns around delayed surgeries due to waiting on sterile processing departments (SPD), significantly reduces surgical site infection (SSI) risk, lessens spiraling logistics and freight expenses, and reduces general high cost of sales per transaction born by the implant OEMs.
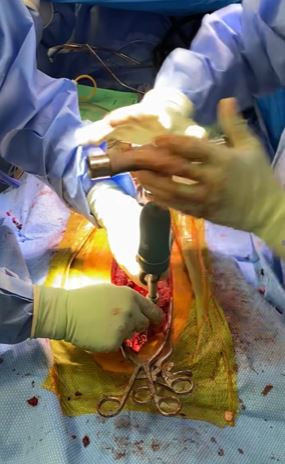
TruTORQ™ Adaptor connected to a reusable ratchet creates a smart instrument providing final precision implant fixation for complex scoliosis cases.
Fenske: Do you encounter sustainability-related concerns with regard to single-use instruments?
Hale: This is a significant opportunity for single-use solutions. In fact, the application of one-way instrument and procedural kits increases sustainability with a 35% reduction in carbon footprint with elimination of SPD and related reprocessing. The existing reusable instrument model requires chemical agents and over 60 gallons of fresh water for cleaning and rinsing every instrument set case and tray, and even more for every steam sterilization. Moreover, there are active recycling programs available today that hospitals and surgery centers are employing to break down and repurpose almost everything in the single-use instrument set, from plastic to stainless steel to packaging materials. Single use is significantly more green than the current reusable instrument model.

Surgeons are enjoying sterile and surgery-ready, single-use instrument sets that are optimized and tailored for various trauma, extremity, and spine procedures. This reduces inventory management and reprocessing at ASCs and hospitals, while improving operating room turnover at a lower carbon footprint than traditional reusable cases and tray systems.
Hale: Our sterile-pack and surgery-ready instrument sets are in high demand to support the full range of trauma and extremity implant cases and, more recently, spine and large joints. The benefits are sterile implants and sterile instruments are always available to support a case. It simplifies the logistics scenerio with a one-way instrument model and it simplifies the surgery with a paint-by-numbers approach to laying out the procedural kit. Overall, it is reducing waste in the supply chain and speeds up operating room turnover. For surgeons, they can perform more surgeries in one day as they don’t have to wait for sterile processing at their hospital or ASC. The instruments and implants are always ready and always sterile; that’s why we call them Surgery Ready™ kits.

ECA Medical’s new TruTORQ™ and TruPWR™ family of single-use and sterile-pack torque limiters range from 0.5 Nm to 14 Nm torque set points with various handles and connectors for use across virtually all ortho and spine procedures.
Fenske: How has the increase in ASCs impacted orthopedic device development?
Hale: More than 68% of all surgeries have or are moving to the ASC with orthopedic surgeries accounting for 20% or more of those cases; they are among the most profitable for surgeons and ASC owners. This shift has accelerated the demand for our single-use instruments and the partnership we have with many of the industry’s leading implant OEMs to tailor a family of modular implant kits across the trauma, extremity, spine, and large joint segments. As part of our Surgery Ready Made Easy™ program, we provide the know-how and expertise gained from 44 years of producing single-use solutions.
The single-use or one-way instrument model offers between $200 to $1,400 (or more) in savings per case, as well as reduced risk of SSI with fresh and sterile-pack instruments. They are also a force multiplier, freeing up sales reps to be out selling or in a case supporting their surgeon customer instead of serving as logistics clerks, moving cases and trays from site to site hours or days before a case. With reusable tray sets costing from $15,000 to $100,000 (depending on the procedure), this reusable model is cost prohibitive to cover the ASC growth. The single-use procedure kit model is part of the solution to best serving the ASCs.

Implant screws and plates can come loose, requiring costly revision surgeries as a result of uncalibrated reusable torque limiters. An alternative solution is realized with single-use torque limiters that are clinically robust, providing precision fixation for every implant and patient.
Fenske: Do the products sold to ASCs differ from those sold to hospitals and if so, how?
Hale: The surgery-ready instrument sets or stand-alone instrumentation is identical for both settings. This is important as it reduces the number of SKUs and makes fulfillment and product tracking easier. Optimizing the instrumentation footprint and packaging is critical as is creating an efficient transfer to the sterile field and the surgical process flow in the operating room. We consider all these dynamics and needs when we bring a sterile-pack instrument or procedural kit to market.
We actually have hospital systems calling us and wanting to order our procedural kits directly (although we redirect them to our customers as we do not sell direct). They are finding that our single-use kits are more efficient than the reusable instruments they are currently using. They are also doing this to reduce their liability for SSIs.

ECA’s novel TruPWR™ single-use torque limiter connects to popular power tools from Stryker and CONMED, and can be used to complete a complex scoliosis case with one instrument providing perfect implant fixation. TruPWR reduces surgeon fatigue and OR time, and provides final fixation for spine and large joint implants.
Hale: There is a change quietly taking hold that will have dramatic impact on the industry—changes to the fulfillment or delivery model for instruments and implants to the customer site and operating room, especially in the fast growth outpatient setting. There are huge logistics hassles, reprocessing, inventory management, and support costs in this area with many being identified and addressed during the recent pandemic. This has created new opportunities to employ and accelerate the application of single-use or one-way instruments and surgery-ready implant fixation kits to reduce those costs and improve overall efficiencies and value to customers.
In addition, technology such as RFID can be used to track the procedural kits in the field and improve fulfillment time and reduce lost inventory. This not only improves the bottom line and reduces the headache for the operations teams, but it also makes the sales reps more effective in the field as they do not have to spend as much time dealing with inventory issues.
Fenske: Do you have any additional comments you’d like to share based on any of the topics we discussed or something you’d like to tell orthopedic device manufacturers?
Hale: The entire lifecycle of the single-use delivery system makes healthcare more sustainable than the current reusable system used today. It is more sustainable in four ways—functionally, financially, environmentally, and safety.
- Functionally, the nature of the single-use delivery system offers pristine, calibrated instruments every time as they are only used during that one surgery. The improvements in resins and engineering have made these instruments more robust than historical perception of plastic instruments.
- Financially, the improved efficiencies, lowered logistics costs, elimination of reprocessing, and other factors have resulted in savings of $200 to $1,400 per case through the use of single-use instrumentation.
- Environmentally, multiple studies have shown single-use instrumentation has a much lower carbon footprint than reusable instrumentation.
- Safety, using reusable instruments increases the risk of contracting SSIs by two to three times.
Click here to learn more about ECA Medical >>>>>