Quality, Recalls & Risk
- All News
- Breaking News
- Certifications
- Clinical Trials
- Device Approvals & Patents
- Financial & Deals
- International
- Market Data & Trends
- ODT Best Bets
- OEM News
- Orthopedic Innovators
- People
- Product & Service Releases
- Products
- Quality, Recalls & Risk
- Regulatory & Legal
- Supplier & Contract Mfg.
- Videobites
-
Biologics

Aziyo Biologics Recalls One Lot of FiberCel Fiber Viable Bone Matrix
Some patients that tested positive for tuberculosis after treatment.Michael Barbella, Managing Editor 06.07.21
-
Extremities

FDA Advises Plastic Component Could Break in Stryker STAR Ankles
Higher than expected risk of the device's polyethylene component breaking was found.Sam Brusco, Associate Editor 03.16.21
-
MIS/Robotics | Spine/Neurology

Zimmer Biomet Recalls ROSA Brain 3.0 Robotic Surgery System
The device is being recalled due to a software issue that incorrectly positions the robotic arm.U.S. Food and Drug Administration 11.08.19
-
Software & Quality

2019: The Year to Enhance Quality in Orthopedic Product Development
...Anthony Parise, Product Strategist—Life Sciences, ETQ 02.13.19
-
Large Joint
Past Dues: A Review of Industry in 2018
The industry confronted old ghosts in 2018 as it defended itself against a documentary and crammed for regulatory changes.Michael Barbella, Managing Editor 11.30.18
-

MDSAP Audits Update: Progress, Challenges, and a Cost Reduction Case Study
...Anthony Parise, Product Strategist—Life Sciences, ETQ 11.30.18
-

Data Monitoring Committees: Making DMCs Work for Your Study
...Margeaux Rogers, Senior Associate, Regulatory Affairs, Musculoskeletal Clinical Regulatory Advisers LLC 11.30.18
-

Top 10 Findings from a Warning Letter Analysis
...Linda Braddon, Ph.D., President and CEO, Secure BioMed Evaluations 11.30.18
-
Biocompatibility & Testing | Software & Quality

AAOS: GE Additive Launches Orthopedic Validation Consultancy
Q&A with Anders Ingvarsson, product manager of Arcam EBM detailing release of a new solution at the AAOS 2018 annual meeting.GE Additive 03.09.18
-

DJO Notifies Individuals of Potential Breach of Personal Information
Affects those who received a DJO product from a Las Vegas hospital between July 17—Oct. 16, 2017.Business Wire 01.09.18
-
Large Joint
Stryker Hip Lawsuits Move Forward in New Jersey
Issuance of 4th Case Management Order governing LFIT V40 Femoral Head claims, Bernstein Liebhard LLP reported.PR Newswire 11.27.17
-
Software & Quality

Managing Deviations and Adequately Recording Failures
...Emily Ysaguirre, Content Marketing Writer, EtQ 11.21.17
-

A Risk-Based Approach to QMS: ISO 13485:2016 Requirements
...Bryan Brosseau, RAC, Vice President, Quality and Regulatory Affairs, Secure BioMed Evaluations 11.21.17
-
Spine/Neurology | Trauma/Sports

NASS News: New Standard Supports High-Strength Alloy for Surgical Implants
The new alloy does not contain elements such as nickel, chromium, or cobalt.ASTM International 10.13.17
-

Comparing CGMP Pharma vs. Device: Subpart A—General Provisions (Part II)
...James A. Dunning, Owner, QPC Services LLC 10.02.17
-
Bracing/Prosthetics | Software & Quality

PLM Software Offers a Step Forward in Prosthetics Development
BionX meets time-to-market, compliance, and efficiency goals to produce the world’s only active prosthetic foot.Online Exclusives Alaine Portnoy, Senior Marketing Manager, Omnify Software 09.06.17
-
Software & Quality

4 FAQs: Medical Device Single Audit Programs
...Alexa Sussman, Content Marketing Writer, EtQ 08.15.17
-
Coatings/Surface Modification | Materials
Scaffold Breakthrough May Banish Implant Infections
Cross-linked collagen scaffold marks an important step forward against a major health problem.IOP Publishing 06.20.17
-
Large Joint
Reducing Infections in Total Hip & Knee Replacement Patients
Rheumatologists & orthopedic surgeons collaborate to provide new recommendations for perioperative management.American College of Rheumatology (ACR) 06.19.17
-
Spine/Neurology
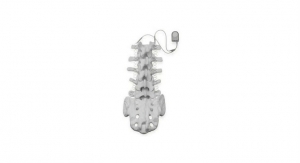
Zimmer Biomet Recalls Implantable Spinal Fusion Stimulators
The devices were recalled due to potential of harmful chemicals which may be toxic to tissues and organs.U.S. Food and Drug Administration 06.02.17













