Columns
-

Supply Chain Simplicity 101 at the ODT Forum 2024
The complexities of supply chain management was among the major topics of conversation at this year's ODT Forum.Michael Barbella, Managing Editor 05.21.24
-
Software & Quality

The Future of Artificial Intelligence in Orthopedics
AI-assisted review of implanted devices before a revision procedure is seeing promise.Ilsa Webeck, Managing Director & Founder, MedTech Strategies 05.21.24
-

Process and Design Looping: Total Product Lifecycle
This looping process isn’t paper-pushing work; it brings value.Meredith P. Vanderbilt JD, RAC, CQA, MSE, BSE, Director of Consulting, Empirical 05.21.24
-
Materials

FDA Regulatory Challenges for Magnesium-Based Orthopedic Devices
Magnesium and its alloys are considered the next-generation biodegradable biomaterial for orthopedics.Mehdi Kazemzadeh-Narbat, Ph.D., PMP, CQA, Director, Regulatory Affairs, MCRA LLC 05.21.24
-
Coatings/Surface Modification

Titanium Implant Textures Rely on Lasers
Laser texturing on functional titanium implant surfaces can help speed bone growth and provide other benefits for patients.Erik Poulsen, Medical Market Segment Manager, GF Machining Solutions 05.21.24
-

The Innovation Pipeline: How Patents Drive Growth in Orthopedics
Patent applications offer a unique glimpse into a company's research and development pipeline.Eric Heinz, Founder and CEO, Heinz Ventures 05.21.24
-
3-D Printing/Additive Mfg.

Empowering Contract Manufacturing Growth with 3D Printing
The most innovative contract manufacturers embrace additive manufacturing alongside traditional manufacturing technologies.Aaron Schmitz and John Ruggieri, 3D Systems and ARCH Medical Solutions 05.20.24
-
Spine/Neurology

Onward and Upward in Treating Spinal Cord Injury
Onward Medical's ARC therapy delivers targeted, programmed spinal cord stimulation to restore movement and other functions in people with spinal cord injury.Sam Brusco, Associate Editor 03.19.24
-
Large Joint

U.S. Obesity Versus Hip and Knee Replacements
The connection between the increase in weight is correlated to an increase in annual medical costs.Ilsa Webeck, Managing Director & Founder, MedTech Strategies 03.19.24
-

How Medical Manufacturers Can Practice Due Diligence for Change Control
A broader view of design and process changes and the implications of those changes is critical to both the product’s and company’s continued success.Meredith P. Vanderbilt, JD, RAC, CQA, MSE, BSE, Director of Consulting, Empirical 03.19.24
-
3-D Printing/Additive Mfg.

Boost Mobility: Precision Joint Replacement with 3D-Printed Implants
3D printing enables manufacturers and surgeons to approach arthroplasty in a whole new way.Dr. Gautam Gupta, SVP & General Manager, Medical Devices, 3D Systems 03.19.24
-
Biologics

CMC Considerations for Medical Device-Led Combination Products
Creating antibiotic-loaded orthopedic combination products demands meticulous yet distinct CMC considerations.Nicole Gribbin, Associate Director of Regulatory Affairs, Pharmaceuticals and Biologics, MCRA 03.19.24
-

Industry Dynamics from AAOS 2024
A closer examination of some key orthopedic device industry battlegrounds.Florence Joffroy-Black and Dave Sheppard, MedWorld Advisors 03.19.24
-

Payoff Pitch Playoff Debuting at AAOS 2024
OrthoPitch is billed as an opportunity for companies to demonstrate new products and solutions to industry experts.Michael Barbella, Managing Editor 02.05.24
-
Software & Quality

What Is the Future and Value of AI in Orthopedic Technology?
AI deployment could release latent value equivalent to 2.6% to 4.5% of annual revenues.Maria Shepherd, President and Founder, Medi-Vantage 02.05.24
-

Intro to Due Process for Orthopedic/Medical Product Makers
Delving into critical aspects of the expected processes for medical product manufacturing lifecycle management.Meredith P. Vanderbilt JD, RAC, CQA, MSE, BSE, Director of Consulting, Empirical 02.05.24
-

3 Strategies to Help Surmount Orthopedic Talent Scarcity
Companies have options to acquire the people resources they require, provided they're strategic in their approach.Tania de Decker, Managing Director—Global Strategic Accounts, Randstad Enterprise Group 02.05.24
-

The Contract Manufacturer’s Role Evolves from Transactional Associate to Strategic Partner
An in-depth and uncompromising partner selection process will be fundamental for success as an orthopedic device OEM.Neal Walters, Partner, Kearney 02.05.24
-
FDA Looks to Industry for Collaboration and Harmonization
The FDA will no longer be a member of the Global Harmonization Working Party (GHWP).Rod Mell, Executive Head—Medical Devices, Regulatory Compliance Associates 02.05.24
-
3-D Printing/Additive Mfg.
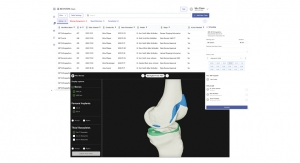
Benefits of Automated Plan-to-Print Workflows for Patient-Specific Devices
Patient imaging data segmentation and 3D modeling design software are expediting design and development of patient-specific implants and instrumentation.Ben Johnson, VP, Portfolio & Regulatory, Healthcare, 3D Systems 02.05.24













