Solvay11.10.17
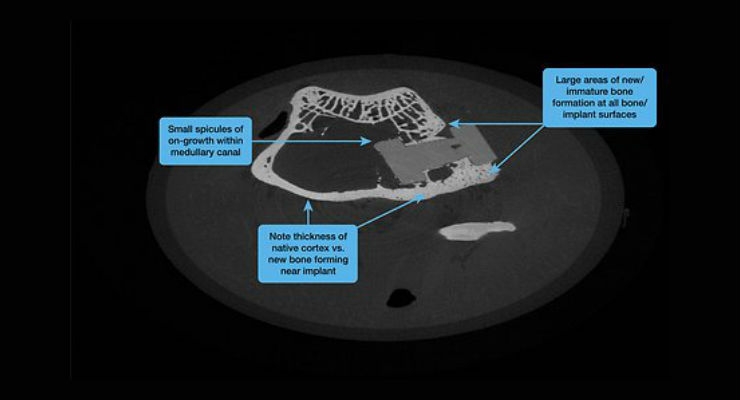
Solvay, a global supplier of specialty polymers, announced that medical device-maker DiFusion Technologies chose Zeniva ZA-500 polyetheretherketone (PEEK) as the base polymer for its ZFUZE osteoconductive PEEK composite for spinal implants. The new compound exhibited large areas of new bone formation on all bone implant surfaces in recent testing by DiFusion, who shared its results at the recent NASS 2017 event in Orlando.
PEEK is an attractive alternative to titanium for spinal implants because it shares similar modulus to bone, and its radio transparency allows for easy visualization in X-rays. The polymer is also inert, which means it does not interact with human tissue. While this quality supports biocompatibility, it means that PEEK does not naturally lend itself to bone growth. DiFusion solved this problem by compounding negatively charged zeolites into Solvay’s Zeniva PEEK polymer.
“It was sort of a penicillin moment,” said Derrick Johns, CEO of DiFusion Technologies. “We started out engineering anti-microbial polymers by first loading zeolite particles with silver before compounding them. But we discovered if we took the silver cations out of the zeolite, they imbued PEEK with a negative charge. Osteoblast cells are attracted to the negatively charged surface at a far higher rate than titanium, and yet we were able to preserve the polymer’s outstanding visualization, modulus and strength benefits.”
Solvay was an early collaborator in the development of DiFusion’s patented ZFUZE composite, combining industry-leading materials expertise with technical and regulatory support for medical devices. Zeniva ZA-500 PEEK was of particular interest to DiFusion due to the polymer’s higher flow, which facilitates both the compounding process and the extrusion of osteoconductive implants.
“In addition to our materials expertise, Solvay’s open innovation business model was instrumental to the successful innovation of DiFusion’s ZFUZE osteoconductive composite,” said Jeff Hrivnak, global business manager for Healthcare at Solvay’s Specialty Polymers Business Unit. “Our uniquely collaborative approach to customer projects differentiates us from other PEEK suppliers in this industry, and it allowed us to pool our respective capabilities and resources with DiFusion to solve this demanding challenge.”
DiFusion’s ZFUZE composite technology is in the final stages of its 510(k) approval process with the U.S. Food and Drug Administration. It is expected to be commercially available in the U.S. early next year.
PEEK is an attractive alternative to titanium for spinal implants because it shares similar modulus to bone, and its radio transparency allows for easy visualization in X-rays. The polymer is also inert, which means it does not interact with human tissue. While this quality supports biocompatibility, it means that PEEK does not naturally lend itself to bone growth. DiFusion solved this problem by compounding negatively charged zeolites into Solvay’s Zeniva PEEK polymer.
“It was sort of a penicillin moment,” said Derrick Johns, CEO of DiFusion Technologies. “We started out engineering anti-microbial polymers by first loading zeolite particles with silver before compounding them. But we discovered if we took the silver cations out of the zeolite, they imbued PEEK with a negative charge. Osteoblast cells are attracted to the negatively charged surface at a far higher rate than titanium, and yet we were able to preserve the polymer’s outstanding visualization, modulus and strength benefits.”
Solvay was an early collaborator in the development of DiFusion’s patented ZFUZE composite, combining industry-leading materials expertise with technical and regulatory support for medical devices. Zeniva ZA-500 PEEK was of particular interest to DiFusion due to the polymer’s higher flow, which facilitates both the compounding process and the extrusion of osteoconductive implants.
“In addition to our materials expertise, Solvay’s open innovation business model was instrumental to the successful innovation of DiFusion’s ZFUZE osteoconductive composite,” said Jeff Hrivnak, global business manager for Healthcare at Solvay’s Specialty Polymers Business Unit. “Our uniquely collaborative approach to customer projects differentiates us from other PEEK suppliers in this industry, and it allowed us to pool our respective capabilities and resources with DiFusion to solve this demanding challenge.”
DiFusion’s ZFUZE composite technology is in the final stages of its 510(k) approval process with the U.S. Food and Drug Administration. It is expected to be commercially available in the U.S. early next year.