Medtronic plc01.09.18
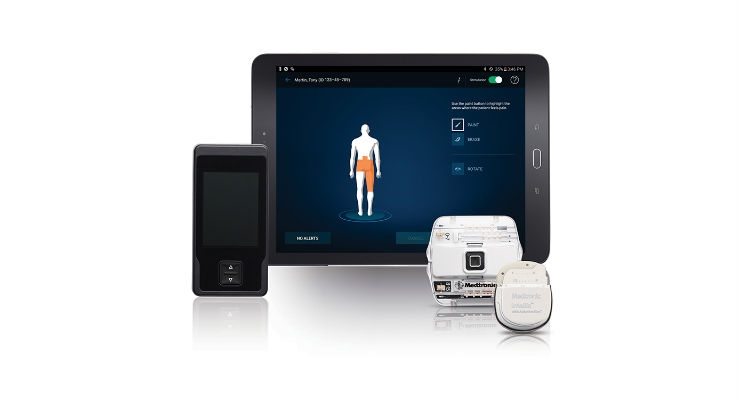
Medtronic plc today announced the first patient enrolled in the Vectors Post Market Clinical Study. The study will follow patients with chronic intractable pain who are undergoing spinal cord stimulation (SCS) treatment managed with the EvolveSM workflow,* which standardizes guidance that balances high-dose (HD) and low-dose (LD) therapy settings to help physicians optimize treatment. Evolve runs on Medtronic SCS systems including Intellis, the world’s smallest implantable SCS neurostimulator, which recently received U.S. Food and Drug Administration and CE Mark approval. The first patient was enrolled by The Center for Interventional Pain & Spine in Wilmington, Del.
“Our goal is to improve or restore function. Even with all of today’s technological advances, chronic pain can be challenging to manage; this is further complicated by the opioid crisis,” said Michael Fishman, M.D., from The Center for Interventional Pain & Spine. “Standard treatment guidance has the potential to help optimize pain relief, and the Vectors Post Market study will provide valuable data about the efficacy of high dose stimulation using standardized programming through the Evolve workflow.”
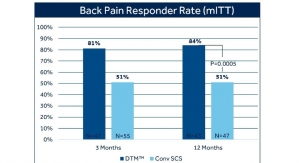
The Vectors study is a prospective, multi-center, post-marketing study that will enroll up to 175 patients with chronic intractable pain of the low back and legs at up to 25 sites in the United States. The study will assess SCS’s long-term efficacy and impact on quality of life and was designed to provide evidence for the Evolve workflow by evaluating the effectiveness and potential patient benefits of having access to both HD and LD stimulation modalities. Patients will be followed for 12 months post implant.
“The Vectors study will generate meaningful data about the use of the Evolve workflow and will help further physicians’ understanding of how to use this simple, versatile approach to enable effective, long-term pain relief with SCS,” said John Hathaway, M.D., Northwest Pain Care of Spokane, Wash., and primary investigator. “Knowing how to best use non-opioid treatment options—like the Intellis SCS platfor
“Our goal is to improve or restore function. Even with all of today’s technological advances, chronic pain can be challenging to manage; this is further complicated by the opioid crisis,” said Michael Fishman, M.D., from The Center for Interventional Pain & Spine. “Standard treatment guidance has the potential to help optimize pain relief, and the Vectors Post Market study will provide valuable data about the efficacy of high dose stimulation using standardized programming through the Evolve workflow.”
The Vectors study is a prospective, multi-center, post-marketing study that will enroll up to 175 patients with chronic intractable pain of the low back and legs at up to 25 sites in the United States. The study will assess SCS’s long-term efficacy and impact on quality of life and was designed to provide evidence for the Evolve workflow by evaluating the effectiveness and potential patient benefits of having access to both HD and LD stimulation modalities. Patients will be followed for 12 months post implant.
“The Vectors study will generate meaningful data about the use of the Evolve workflow and will help further physicians’ understanding of how to use this simple, versatile approach to enable effective, long-term pain relief with SCS,” said John Hathaway, M.D., Northwest Pain Care of Spokane, Wash., and primary investigator. “Knowing how to best use non-opioid treatment options—like the Intellis SCS platfor